There is a lot of debate about whether feeding raw bones is potentially dangerous for dogs and cats. People worry that feeding raw meat to their pets increases the risk of food poisoning and salmonella infection. To make sure that fears are in vain, it is enough to study information about the nature of the stomach environment of dogs and cats. The stomach environment of animals is directly related to their diet.
High acidity of gastric juice
The stomach acidity (or stomach pH) of dogs and cats fed natural foods is very high (i.e., the stomach juice is very acidic). Normal gastric acidity is 2 or less. This value depends on how much animal protein the pet receives.
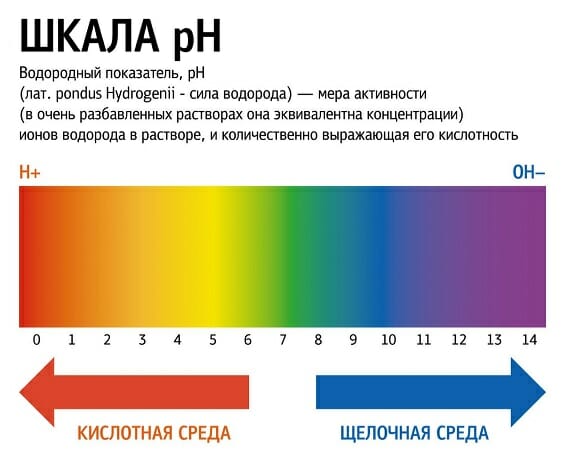
The lower the pH, the more acidic the environment
Due to their high acidity, raw meat and bones are easy to break down and digest. High acidity effectively destroys bacteria. This is especially true for pathogenic bacteria:
- salmonella,
- clostridium,
- campylobacter,
- coli.
The specific stomach environment of dogs and cats is formed as a result of their natural diet. It helps animals digest meat and bones. High acidity protects pets from diseases because it kills harmful microorganisms. In other words, the digestion process occurs in accordance with the needs of predators, which are our pets.
Hydrochloric acid in the stomach: what functions it performs, methods for normalizing pH
There are substances in the human body that perform important digestive functions. One of the components is hydrochloric acid in the stomach. This is a product secreted by the main glands of the fundus. A change in its homeostasis leads to a deterioration in the patient’s condition and disruption of his quality of life.
What is hydrochloric acid and how is it produced?
In order to fully understand the functional role of hydrochloric acid in the stomach, it is necessary to study the entire process.
Digestion begins when the thought of food arises and its smell is felt. Receptors are activated, the central nervous system centers are activated, and information about the upcoming act of eating is provided. As a result, the fundic glands become aware of the need for gastric juice. This is the first phase of secretion. The stomach prepares for food intake by secreting a meager amount of enzymes.
After eating food, these impulses intensify, and much more secretion is released.
Parietal cells, thanks to chemoreceptors, capture information about the reaction of the environment and regulate it by releasing acid.
The second phase of secretion is the most basic and depends directly on the release of gastrin. It stimulates glandular cells and provokes maximum release of hydrogen chloride during the act of eating.
The final phase occurs thanks to somatostatin. It is released into the stomach after a signal that food has entered the duodenum.
The stretching of the stomach and the pressure on the receptors becomes less, the need for secretion of gastric juice decreases. Somatostatin deactivates the cells of the fundus of the stomach, and acid secretion decreases to a minimum level.
Once in the duodenum, the pH becomes alkaline due to neutralization by bile.
Functions of hydrochloric acid
Hydrogen chloride converts pepsinogen into an active compound necessary for the digestion of chyme. Its function is to break down proteins into short amino acid chains. The enzyme requires an optimal acidic environment for normal metabolism.
The digestive function of hydrochloride compounds is the ability to break down protein molecules into amino acids and denature proteins. When dairy products enter the stomach, they curdle and casein is formed along with pepsins and chemosins.
Protein Denaturation
Denaturation is the process of transforming the globular structure of a protein into a simple one. Initially, protein consists of amino acids connected in series. Next, disulfide bonds are formed between the chains, and it conforms (twists) into a compact structure - a globule. More often, these are tertiary and quaternary forms. This shape is explained by the need to correctly position the long chain.
For normal energy metabolism and obtaining important elements for structuring the protein structures of the human body. When exposed to acid, disulfide bonds are the first to break. The structure returns to the original serial circuit. It comes apart piece by piece, like a mosaic, and is included in processes (formation of RNA, muscle fibers, oxidation to produce energy).
Acidity as an indicator of stomach condition
The concentration of hydrochloric acid in the stomach not only shows how ready the organ is to eat food, but also regulates normal processes. Normally, the gastric mucosa is covered with secretion from the antral glands. This is a protective mucus. It can withstand a certain pH. The secretion is constantly produced to maintain the integrity of the mucosa and block the coagulation effect on the endothelium.
Free hydrochloric acid
The composition of gastric juice includes dissociated hydrochloric acid. It is written this way – H+ and Cl-. The study of its amount after a test meal is 20-40, 0.07-0.14% of the absolute concentration. This is an inactive form.
Bound hydrochloric acid
It is not a dissociated species associated with a specific protein. This is a compound that can interact with active substances and absorb essential nutrients. The reaction of the compound is less acidic than that of the bound acid.
Methods for studying the acidity of gastric juice
To check, intragastric pH-metry, or fractional sounding, is used. To study acidity, phenolphthalein, dimethylaminoazobenzene and alizarine sulfonic acid indicators are used. Phenolphthalein, when the pH shifts to the alkaline side, acquires a characteristic pink or crimson color.
Dimethylaminoazobenzene stripes turn red if the environment is acidic and free hydrogen chloride predominates. An increased concentration of proteinized hydrochloric acid is indicated by orange color.
Acid-dependent diseases of the gastrointestinal tract
A healthy body has strong protection and homeostasis, thanks to which normal digestive functions are performed. The first and most famous disease associated with changes in acidity is gastritis. The mucous secretion cannot properly protect the mucosa from the effects of pathogens. This occurs due to:
- disruption of antral cell secretion;
- changes in the composition of mucus;
- distortion of normal HCl release;
- constant intake of acidic foods.
Signs, causes and consequences of increased acidity
Regulating acidity is an independent process. The body reacts to any change in a positive or negative direction by activating its defense systems. Increased acidity occurs when it cannot accurately control secretions.
The first symptoms are heartburn, sour belching, and hungry abdominal pain. They occur as a result of gastritis, poor diet, peptic ulcers, large amounts of Helicobacter pylori, and alcoholism. Increased acidity can significantly reduce a person's quality of life.
Signs, causes and consequences of low pH
A decrease in acidity is caused by systematic overeating, fasting, poor diet, stress, sympathoactive nervous regulation, lack of vitamins, in particular PP and B1, and lack of zinc. Violation of concentration leads to a distortion of the optimal environment, opportunistic microflora multiplies, and the body becomes infected.
Along with this, insufficient activation of enzymes causes abnormal digestion. The disease causes iron deficiency anemia, a lack of B12, C, A, and beneficial elements.
pH normalization methods
There are two types of effects: neutralizing the pH and changing the rate and amount of HCl release. Reducing pH with antacids, “ Pechaevskie ”, “ Rennie ”, “ Phospholugel ”. A solution of baking soda can be used in everyday life, but when the acid is neutralized, CO2 is formed, which bloats the stomach and can lead to pain and severe belching.
To normalize the endocrine level, H2-histamine receptor blockers and proton pump inhibitors are used: Omeprazole , Dexlansoprazole , Esomeprazole .
Source: https://GastrituNet.online/bolezni-zheludka/stroenie/fiziologiya/solyanaya-kislota-v-zheludke.html
Carnivores have a short gastrointestinal tract
The time it takes to digest food depends on the length of the gastrointestinal tract. In carnivores, including dogs and cats, it is short. Humans have a gastrointestinal tract of medium length, and herbivores have the longest gastrointestinal tract. Based on the length of the gastrointestinal tract, one can judge which diet is best suited for a particular animal. The short gastrointestinal tract of dogs and cats indicates that they have a high rate of digestion. Therefore, like all carnivores, they are best suited to a natural meat diet. Carnivores digest fresh meat in 8-12 hours. Herbivores digest plant food in 3-5 days.

Hydrochloric acid in the stomach: functions and significance
The secretion of gastric juice occurs through the work of the gastric mucosa. It is a colorless, odorless liquid with small lumps of mucus.
Any deviations from this norm, such as changes in color and thickness, indicate problems with the gastrointestinal tract. The composition of gastric juice is complex, since it is produced by various cells of the gastric mucosa.
Its main component is hydrochloric acid, which, in turn, has a concentrated composition.
Composition of gastric juice
In addition to hydrochloric acid, gastric juice contains the following components
- Bicarbonates (they neutralize the harmful effects of hydrochloric acid on the walls of the stomach).
- Pepsinogen, which turns into pepsin (the latter is responsible for the breakdown of proteins). Pepsin is divided into another family of enzymes, each of which has its own functions.
- Mucus (it also protects the mucous membrane from destruction).
- Castle factor (an enzyme that helps absorb B12).
However, the main component of gastric juice is still hydrochloric acid. This is what we will talk about.
What is hydrochloric acid?
It is produced by the parietal cells of the gastric glands, located on the body and bottom of the organ. In essence, the mucous membrane is divided into several zones: one produces hydrochloric acid, the other secretes bicarbonates that neutralize it. It is noteworthy that men have several times more parietal cells than women.
other acids in the stomach are insignificant. So, if lactic acid is found in it, this indicates that hydrochloric acid is produced in small quantities (low stomach pH) or not produced at all. The latter may indicate such serious failures as oncology.
Hydrochloric acid in the stomach has a strict concentration level - it is 0.3-0.5% (or 160 mmol/l). Its composition is so concentrated that if there were no protective substances in the gastric juice and mucous membrane, it would burn out its own stomach.
That is why, when there is insufficient production of mucus by the stomach, a person develops gastritis or duodenal ulcers. Acid is constantly present in the stomach, but its amount increases in response to food intake.
Basal secretion of hydrochloric acid (that is, morning) is 5-7 mmol/hour.
A healthy stomach produces up to 2.5 liters of hydrochloric acid per day!
The secretion of hydrochloric acid has 3 phases.
- Reaction to the taste and smell of food. It is triggered and transmitted from the central nervous system to gastric cells through nerve endings.
- After food enters the body, a more significant phase begins. Gastrin acts on parietal cells, stimulating the production of hydrochloric acid.
- The final phase begins after chyme (already digested food) enters the duodenum. Due to an increase in hydrochloric acid, the stomach produces somatostatin, an acid blocker.
What functions does hydrochloric acid perform in the stomach?
First of all, it improves digestion, destroys most bacteria that enter the stomach with food, which slows down or even interferes with the putrefactive process.
What are the functions of hydrochloric acid in the stomach? Below is a list that details this issue.
- Denaturation of proteins (this is the destruction of their molecular structure) and their swelling.
- Activation of pepsinogen, which turns into pepsin - one of the most important enzymes that break down proteins.
- Creating an acidic environment under which enzymatic digestion occurs much easier.
- Evacuation of food from the stomach to the duodenum, where digestion continues.
- Antibacterial effect - many bacteria cannot live in such an aggressive environment.
- Stimulation of pancreatic juice secretion.
The role of hydrochloric acid in the breakdown of proteins deserves special attention. The importance of proteins in the body is enormous. This question has been studied by scientists for many decades.
It has been established that hydrochloric acid in the stomach stimulates the production of pepsin, creating a favorable environment for its activity, and promotes partial denaturation and swelling of proteins.
In the duodenum, hydrochloric acid stimulates the production of secretin, improves iron absorption and has a bactericidal effect.
Proteins and acidity of gastric juice
The role of hydrochloric acid in the digestion of proteins is still unclear. However, it has been established that with inflammatory diseases of the stomach, its secretion and, as a consequence, the digestion of proteins are disrupted.
The importance of proteins in our body can hardly be overestimated. This group is divided into many subgroups, each of which does its own thing. Thus, hormone proteins control life processes (growth and reproduction), enzyme proteins provide chemical processes (respiration, digestion, metabolism), hemoglobin saturates cells with oxygen.
Denaturation of proteins (this facilitates the process of their subsequent breakdown) allows the body to use their properties to the maximum. Every protein is made up of amino acids. Most of them are synthesized by our body, but there is a group of so-called essential amino acids that enter the body only from the outside.
Gastric acidity
Such an important aspect as the pH of the stomach directly depends on hydrochloric acid. And if there is a deviation from the norm, gastritis, dyspeptic disorders and other unpleasant conditions occur. Acidity in the stomach can be low, normal or high.
https://www.youtube.com/watch?v=4IJUTugYtcI
Despite the “popularity” of increased pH, people often have low or normal acidity. The latter ranges from 0.8 to 1.5.
Low stomach acidity
Low acidity occurs with constant stress and inflammatory diseases. This happens due to the stimulation of the sympathetic nervous system, which directly affects the production of gastric juice.
A decrease in acidity leads to worse digestion of food and stomach spasms. Food remains in the cavity and begins to rot, increasing the proliferation of pathogenic bacteria. A person suffers from flatulence and nausea. The latter is a response to stomach spasms.
Moreover, the process of absorption of all nutrients contained in our food is actively disrupted, which leads to disruption of the functioning of the entire body. By the way, it is precisely because of the natural decrease in pH that after 40 years a person begins to age rapidly.
That is, hydrochloric acid in the stomach actually affects the health of the entire body.
The stomach, surprised by the excessive proliferation of bacteria, begins to turn on its protective function, resulting in inflammation. It is treated with drugs that further inhibit the production of hydrochloric acid - and the circle closes. A person is forced to constantly visit a doctor.
Even heartburn, which we are accustomed to considering as a consequence of an increase in the amount of gastric juice, is considered only a product of acetic acid fermentation.
In a diseased stomach, lactic acid begins to actively form. Due to the inability of the stomach to produce enough mucus, it damages the walls of the organ. In such cases, a diagnosis of gastroduodenitis is made.
Parasites and low stomach acidity
Parasites cannot live in a healthy stomach (however, this does not exclude their localization in other organs and systems of the body), since they are literally burned by hydrochloric acid.
But as soon as it decreases, colonies of parasites begin to flourish, causing extremely unpleasant symptoms.
The absorption of nutrients is further impaired, and there is a risk of food allergies (if the parasites “did not like” the food they consumed).
Increased stomach acidity
Despite the opinion of many gastroenterologists, high acidity is much less common than low acidity.
The danger is that with prolonged hypersecretion of gastric juice, ulcers of the esophagus and stomach appear. The patient is bothered by heartburn and pain.
This is where proton pump inhibitors - Omez and its analogues - will be useful. Symptoms are relieved with the help of antacids - Gaviscon, Phosphalugel, etc.
To diagnose high acidity, an instrumental examination must be used, because its symptoms can easily be confused with decreased secretion.
How the diet of pets has changed
With the proliferation of thermally processed foods in the pet food industry, the composition of our pets' diets has radically changed. Meat protein is the most expensive component of any feed. To reduce production costs and/or increase profits, it is beneficial to reduce the meat protein content in feed to a minimum. Manufacturers of modern prepared foods have adapted to these financial constraints.
Firstly, they significantly increased the amount of carbohydrates: they added corn, wheat, rice, potatoes and other carbohydrate products. These products are often the first and most basic ingredients in many foods. Secondly, manufacturers replaced expensive animal protein with cheaper plant protein: they added soy and lupine. They increase the total protein content of the feed according to the % on the label, but reduce the cost of producing the feed.
Such product substitution has a bad effect on the gastrointestinal tract of dogs and cats.

Why should acidity remain high?
When animals' diets include food high in carbohydrates and vegetable protein and reduced in animal protein, stomach acidity begins to decrease. The stomach environment becomes alkaline (pH level reaches 4 or higher). Due to a decrease in acidity, digestive problems arise:
- digestion and gastric emptying slow down,
- bacteria and contaminants are destroyed less efficiently,
- raw bones and bone material do not soften, digestive enzymes do not cope: this can lead to obstruction.
These problems become apparent when pets that eat processed food are switched to a natural diet. Since before this they did not receive the necessary proportion of animal protein, their production of stomach acids is reduced. Their gastric juices cannot soften raw bone material and are unable to destroy harmful bacteria.
This is what leads to the unexpected refusal of the gastrointestinal tract from bones and meat. The pet may suffer from vomiting, an attack of acute gastroenteritis, excessive bacterial growth, or an obstruction in the stomach. Delayed gastric emptying encourages the proliferation of any bacteria that survive in the low-acid gastric environment. Because of this, the entire digestion process slows down, and now instead of 12 hours it can take up to 24 hours. This can lead to constipation from excess water reabsorption or loose stools due to the production of short-chain fatty acids in the colon.
What does hydrochloric acid do in the stomach and why is it needed?
What is this liquid needed for and what are the risks of disrupting its production? The importance of hydrochloric acid in the stomach is difficult to overestimate, because it is an important part of the digestive process. Changes in acidity lead to inflammatory diseases of the upper gastrointestinal tract, which require serious drug therapy.
Digestion is a complex function of the gastrointestinal tract, which is regulated by the nervous and endocrine systems. Food processing is carried out in several stages, starting with the oral cavity. The main breakdown of components occurs in the stomach. The main role is played by the secretion, which contains hydrochloric acid.
Why is hydrochloric acid needed in the stomach?
Through the esophagus, crushed food enters the cardial and then the fundic section of the stomach, where gastric juice is secreted. Food is mixed with liquid, which provides several functions. Hydrochloric acid, which is part of the secretion, is needed in the stomach for the following processes:
- converting pepsin and gastricsin into an active form by activating pepsinogen;
- formation of an acidic environment for optimal hydrolysis of protein molecules;
- destroying pathogenic bacteria that enter the body;
- partial denaturation and swelling of proteins for further breakdown by pepsins;
- stimulation of pancreatic juice synthesis;
- maintaining normal motility of the digestive tract.
How is it formed
The process of formation of gastric juice begins before food enters the gastrointestinal tract. The synthesis of hydrochloric acid in the stomach occurs due to neuro-reflex mechanisms with the participation of hormonal factors. The main stages of secretion are shown in Table 1.
Table 1. Secretion phases
| Phase | Actions |
| Cephalic (first) | Acid production begins before food enters the oral cavity with the participation of the visual, olfactory, gustatory, and auditory analyzers. Impulses are transmitted from the central nervous system to the upper gastrointestinal tract. |
| Gastric (second) | When a bolus of food moves into the stomach, the walls of the organ stretch. Gastrin is produced by G cells in the antrum, which ensures the release of histamine followed by the synthesis of hydrochloric acid. |
| Intestinal (third) | It starts after the chyme moves into the duodenum and affects its walls. |
Which glands produce hydrochloric acid
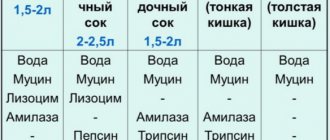
The glands of the stomach produce gastric juice under the influence of hormonal substances and regulators of metabolic processes. The secretion contains several important components that ensure normal functioning of the gastrointestinal tract. The composition of gastric juice is represented by the following substances:
- hydrochloric acid;
- bicarbonates;
- enzymes - pepsinogens, pepsin isoforms;
- mucus;
- Castle factor, an enzyme for the absorption of vitamin B₁₂.
The parietal cells of the gastric glands produce hydrochloric acid in response to the entry of bolus food into the upper digestive tract. It provides the level of acidity that is necessary to break down nutrients and move them into the intestines.
Why hydrochloric acid does not corrode the stomach
The synthesis of hydrochloric acid occurs during the day with varying intensity. A certain amount of secretion is constantly produced. However, it does not corrode the walls of the organ. Let's look at why this happens and what neutralizes hydrochloric acid in the stomach?
Other components of gastric juice provide a protective role. These are bicarbonates and mucus. Accessory cells produce mucus, which concentrates on the surface of the mucous membrane.
Its thickness is up to 6 mm. The gel contains alkaline components in an amount of 45 mmol/l.
Their task is to neutralize the acid of the food bolus, which promotes the opening of the sphincter of the pyloroduodenal zone and the movement of chyme into the duodenum.
Important!
Lack of bicarbonates and mucus gel contributes to increased acidity in the stomach.
Concentration
Hydrochloric acid is needed for digestion and movement of food components through the gastrointestinal tract. It kills bacteria and other pathogenic agents and acts as a kind of antiseptic in the digestive tract. The formation of secretion occurs continuously.
Therefore, the concentration of hydrochloric acid in the stomach remains at a certain level. The amount of substance produced ranges from 5 to 7 mmol/hour. The percentage in gastric juice is 0.3-0.5%.
Women synthesize less acid than men.
How is production regulated?
As food moves forward, it enters first the acid-forming and then the acid-neutralizing zones of the stomach. They are separated by an intermediary zone, which corresponds to the transition of the fundus to the antrum. Therefore, a slightly acidic environment with a pH level of 4.0-6.0 is replaced by a strongly acidic environment, where the pH is less than 3.0.
The main acid-forming function is performed by hydrochloric acid in the stomach. Correction of acid formation is carried out by hormonal and biologically active components as the chyme moves. The fundic glands of the body and antrum secrete acid. The process is regulated with the help of histamine. This substance is produced when the walls of the organ are stretched by food.
Gradually, the concentration of gastric juice increases, which leads to the activation of somatostatin. It blocks the secretion of hydrochloric acid. Another neutralizing mechanism is bicarbonates, which make food alkaline. An optimal environment with low acidity is needed for the reflex opening of the sphincter and the creation of normal peristalsis in the stomach and duodenum.
Attention!
Hypersecretion of hydrochloric acid causes retention of chyme in the stomach; its deficiency leads to its premature entry into the intestine. The process of fermentation and absorption of nutritional components is disrupted.
Causes of hypersecretion
Increased acidity occurs due to exposure to negative factors. The pathology is accompanied by inflammation of the mucous membrane when gastric juice is produced in excess.
The condition is accompanied by unpleasant symptoms characteristic of hyperacid gastritis.
The patient is concerned about:
The main reason is identified when the organ secretes hydrochloric acid in large quantities. This is Helicobacter pylori infection. The pathogenic bacterium is resistant to the acidic environment in the stomach.
It causes an immune inflammatory reaction of the mucous membrane, secretes enzymes that break down mucus, and produces urease, which ferments urea to form ammonia.
As a result, parietal cells are modified, which leads to an excess of secretions and an increased risk of cancer.
Other provoking factors include:
- stress, psycho-emotional stress;
- violation of the nature of nutrition and regime;
- chemical irritants, including some medications;
- genetic predisposition;
- parasitic diseases;
- bad habits - alcohol and smoking tobacco products.
Reasons for shortage
A decrease in the concentration of gastric juice most often occurs against the background of chronic gastritis.
The pathogenic effect of unfavorable factors over a long period of time leads to gradual atrophy of the mucous membrane.
Another reason for decreased secretion release is autoimmune damage to cellular structures. Therefore, there is a lack of hydrochloric acid in the stomach, which is manifested by the following symptoms:
- discomfort and pain in the upper abdomen after eating;
- a feeling of fullness in the projection of the stomach;
- belching with a putrid odor;
- vomiting food eaten;
- bloating;
- alternating constipation and diarrhea.
How can you reduce or increase the concentration of hydrochloric acid in the stomach?
The normal digestive ability of the gastrointestinal tract lies in the coordinated work of the gastrointestinal tract and other body systems.
Why is hydrochloric acid needed in the stomach?
A sufficient level of secretion ensures the process of food processing and prepares chyme for further absorption of nutrients in the intestine.
Inflammatory diseases of the upper digestive tract lead to disruption of the synthesis of gastric juice.
Violation of secretion concentration forms organic and functional changes in the organ. Its lack slows down the breakdown of food components and increases the likelihood of the proliferation of pathogenic bacteria.
When there is a lot of hydrochloric acid, it leads to severe inflammation and destruction of the epithelium.
The medications presented in Table 2 help raise or lower stomach acidity.
Table 2. Main groups of drugs affecting the synthesis of hydrochloric acid
| Reduce pH | Increase pH |
| Proton pump blockers | Natural gastric juice |
| H2-histamine blockers | Stimulators of gastric juice synthesis |
| Antacids |
Hydrochloric acid is an important functional component of digestion. An imbalance in the process of production and neutralization of secretions leads to negative changes requiring treatment.
Recommended materials:
Composition of human gastric juice: the role and significance of the components
Helicobacter pylori bacterium in the stomach and its causes
What diseases are transmitted by kissing with saliva?
What foods are good for the stomach?
What foods cause heartburn?
Source: https://stomach-diet.ru/solyanaya-kislota-v-zheludke/
How to switch to a healthy diet
The body of an animal that eats commercial food cannot immediately adapt to natural food. It takes about 7-10 days to switch your pet to a natural diet. During this time, the acidity level of the stomach will drop to the natural pH level of 2.
Here are some tips for those who want to switch their pet to a healthy diet:
- Transition your pet to a raw food diet gradually. Many cat and dog owners give up on the idea of a raw diet because the animal vomits after eating. From the above it follows that the transition should be gradual, it will take about a week. This period is needed for the acidity of the animal’s stomach to normalize.
- If you plan to feed raw bones (and they are an important part of a healthy diet), include raw meat in your daily diet. Animal protein will keep stomach acidity at the desired level.
- A natural diet will protect your pet from bacterial contamination and food poisoning, and will reduce the risk of obstruction from eating bones. Dogs who eat processed food have significantly higher levels of salmonella bacteria in their feces than dogs who eat raw food.
Remember that cats and dogs have been eating raw meat and bones for 40 thousand years. Eating natural food will not only not harm your pet, but will also make it healthier.