Types of enzymes
- Pepsin. An enzyme is a substance that is produced in the stomach. It acts on protein molecules in food, breaking them down into their elementary components - amino acids.
- Trypsin and chymotrypsin. These substances are part of the group of pancreatic enzymes, which are produced by the pancreas and delivered to the duodenum. Here they also affect protein molecules.
- Amylase. An enzyme is a substance that breaks down sugars (carbohydrates). Amylase is produced in the mouth and small intestine. It decomposes one of the main polysaccharides - starch. The result is a small carbohydrate - maltose.
- Maltase. The enzyme also affects carbohydrates. Its specific substrate is maltose. It breaks down into 2 glucose molecules, which are absorbed by the intestinal wall.
- Saharaza. Protein acts on another common disaccharide, sucrose, which is found in any high-carbohydrate food. Carbohydrates break down into fructose and glucose, which are easily absorbed by the body.
- Lactase. A specific enzyme that acts on the carbohydrate from milk - lactose. When it decomposes, other products are obtained - glucose and galactose.
- Nucleases. Enzymes from this group act on nucleic acids - DNA and RNA, which are contained in food. After exposure, substances break down into individual components - nucleotides.
- Nucleotidase. The second group of enzymes that acts on nucleic acids is called nucleotidases. They decompose nucleotides to produce smaller components - nucleosides.
- Carboxypeptidase. The enzyme acts on small protein molecules - peptides. As a result of this process, individual amino acids are obtained.
- Lipase. The substance decomposes fats and lipids entering the digestive system. In this case, their components are formed - alcohol, glycerin and fatty acids.
Stomach acidity
The main indicator of the normal functioning of the stomach is the level of acidity, that is, the concentration of acid in the gastric juice. This indicator is measured in different parts of the stomach, esophagus and duodenum. Hydrochloric acid in the stomach breaks up complex molecules, which facilitates absorption in the small intestine.
The synthesis of acid in the stomach is less than the established indicators, indicating low acidity. With an increased level of acidity, the acid concentration exceeds the norm. In any case, a shift in this indicator triggers pathological changes in the gastrointestinal tract and causes the appearance of unpleasant symptoms.
Reduced or increased secretion of hydrochloric acid threatens the appearance of chronic gastritis, peptic ulcers and even cancer. Currently, there are a large number of ways to measure acidity levels, but the intragastric method is considered the most accurate and informative. During the day, the concentration of hydrochloric acid is measured simultaneously in several parts of the stomach. This happens with the help of devices that are equipped with special sensors.
Important! Stimulation of gastric juice for research is carried out using products that contain insulin or histamine.
The fractional probing technique is also used. The contents of the stomach are sucked out using a rubber tube. Compared with the previous method, the results of this study are not as accurate. This is due to the fact that biological material is taken from different zones and mixed.
Moreover, the research process itself disrupts the normal functioning of the stomach, and this also distorts the results obtained. Experts distinguish two main types of changes in acidity levels: increased and decreased types. Let's talk about these changes in more detail.
The analysis will show what acid is in the stomach
Increased acidity
Excessive production of hydrochloric acid manifests itself in the form of such unpleasant symptoms:
- heartburn. It usually appears after eating or taking a horizontal position. Heartburn is the result of stomach contents refluxing into the esophagus. Irritation of the mucous membrane is the cause of the burning sensation;
- sour or bitter belching. It appears when gases or food enter the esophagus;
- pain outbreak;
- feeling of heaviness and fullness in the stomach. Even a regular snack causes discomfort;
- decreased appetite;
- bloating;
- rumbling in the stomach;
- nausea, vomiting;
- constipation or diarrhea.
When the production of gastric juice is high, heartburn and an attack of pain occur. If there is high acidity, you should never neutralize it with soda. In the future, this will lead to an even greater increase in the secretion of gastric juice and the formation of deep ulcers on the mucous membrane.
A variety of factors can lead to excessive acidity: dietary errors, bad habits, stressful situations, taking medications. The development of hyperacid gastritis is also based on the influence of Helicobacter pylori infection. This is the only bacterium that is not damaged by hydrochloric acid.
Low acidity
Despite the fact that hypoacid gastritis is much less common, it is considered the most dangerous. A decrease in gastric activity threatens the penetration of pathogenic microorganisms. A decrease in enzymatic properties manifests itself in the form of the following symptoms:
- belching rotten;
- loss of appetite;
- bad breath, which even brushing your teeth cannot eliminate;
- intestinal disorders;
- stool retention;
- an attack of nausea that occurs after eating;
- bloating.
Hypoacid gastritis threatens the development of anemia, hypotension, allergic reactions, and autoimmune processes. A decrease in acidity concentration can even contribute to the development of cancer.
Reduced production of hydrochloric acid can lead to the development of serious pathologies such as anemia, allergies and cancer
Excess digestive enzymes
Excess digestive enzymes are most often observed with a disease such as pancreatitis. The condition is associated with the overproduction of these substances by pancreatic cells and a violation of their excretion into the intestines. In this regard, active inflammation develops in the organ tissue, caused by the action of enzymes.
Signs of pancreatitis may include:
- severe pain in the abdominal area;
- nausea;
- bloating;
- violation of stool character.
A general deterioration in the patient's condition often develops. General weakness, irritability appear, body weight decreases, and normal sleep is disturbed.
Acid-dependent diseases of the gastrointestinal tract[edit | edit code]
The cause of acid-related diseases can be an imbalance in the functioning of the mechanisms of acid production or acid neutralization, insufficient efficiency of the lower esophageal or pyloric sphincters, which is the cause of pathological gastroesophageal and duodenogastric reflux, as well as poor diet or lifestyle. The most important diagnostic factor is the value of acidity in various parts of the organs of the upper gastrointestinal tract (GIT), the change in these values over time. In this case, it is often necessary to know the behavior of acidity simultaneously at several points in the gastrointestinal tract.
How to identify disturbances in the synthesis of digestive enzymes?
- Stool examination. The detection of undigested food residues in the stool indicates a disruption in the activity of the intestinal enzymatic system. Depending on the nature of the changes, it can be assumed which enzyme is deficient.
- Blood chemistry. The study allows you to assess the patient’s metabolic state, which directly depends on the activity of digestion.
- Study of gastric juice. The technique allows you to evaluate the content of enzymes in the stomach cavity, which indicates the activity of digestion.
- Study of pancreatic enzymes. The analysis makes it possible to study in detail the amount of organ secretion, thanks to which the cause of the violations can be established.
- Genetic research. Some enzymopathies may be hereditary. They are diagnosed by analyzing human DNA, in which genes corresponding to a particular disease are found.
Sources[edit | edit code]
- Gorshkov V. A.
Theoretical and clinical aspects of proteolysis in the upper digestive tract. - St. Petersburg, 2005. - 228 p. ISBN 5-7243-0309-5 (erroneous). - Dubinskaya T.K., Volova A.V., Razzhivina A.A., Nikishina E.I.
Gastric acid production and methods for its determination. Tutorial. M.: RMAPO, 2004. - 28 p. ISBN 5-7249-0789-5. - Korotko G. F.
Gastric digestion from a technological perspective - Kuban Scientific Medical Bulletin. 2006, No. 7-8 (88-89), p. 17-22. - Korotko G. F.
Gastric digestion. Krasnodar, 2007. - 256 p. ISBN 5-93730-003-3. - Leya Yu. Ya.
pH-metry of the stomach. - L.: Medicine, 1987. - 144 p. - Linar E. Yu.
Acid-forming function of the stomach in normal and pathological conditions. - Riga, Zinante, 1968. - 438 p. - Sotnikov V.N. et al.
The importance of endoscopic pH-metry in determining the acid-producing function of the stomach / - M.: RMAPO, 2005, 35 p. ISBN 5-7249-0935-9. - Roytberg G. E., Strutynsky A. V.
Internal diseases. Digestive system. Tutorial. - M.: MEDpress-inform, 2007. - 560 p. ISBN 5-98322-341-0. - Rosenfeld L.
Gastric tubes, meals, acids, and analysis: rise and decline / Clinical Chemistry. 1997; 43:837-842. (English)
Basic principles of therapy for enzyme disorders
A change in the production of digestive enzymes is a reason to consult a doctor. After a comprehensive examination, the doctor will determine the cause of the disorders and prescribe appropriate treatment. It is not recommended to deal with pathology on your own.
An important component of treatment is proper nutrition. The patient is prescribed an appropriate diet, which is aimed at facilitating the digestion of food. It is necessary to avoid overeating, as this provokes intestinal disorders. Patients are prescribed drug therapy, including replacement treatment with enzyme preparations.
Specific medications and their dosages are selected by the doctor.
In an adult, about 2-2.5 liters of gastric juice are formed and secreted during the day. Gastric juice is acidic (pH 1.5-1.8). It consists of water - 99% and dry residue - 1%. The dry residue is represented by organic and inorganic substances. The main inorganic component of gastric juice is hydrochloric acid, which is in a free and protein-bound state. Hydrochloric acid performs a number of functions: 1) promotes denaturation and swelling of proteins in the stomach, which facilitates their subsequent breakdown by pepsins; 2) activates pepsinogens and converts them into pepsins; 3) creates an acidic environment necessary for the action of gastric juice enzymes; 4) provides an antibacterial effect of gastric juice; 5) promotes normal evacuation of food from the stomach: opening of the pyloric sphincter from the stomach and closure from the duodenum; 6) stimulates pancreatic secretion. In addition, gastric juice contains the following inorganic substances: chlorides, bicarbonates, sulfates, phosphates, sodium, potassium, calcium, magnesium, etc. Organic substances include proteolytic enzymes, the main role of which is played by pepsins. Pepsins are excreted in an inactive form as pepsinogens. Under the influence of hydrochloric acid they are activated. The optimum protease activity is at pH 1.5-2.0. They break down proteins into albumoses and peptones. Gastricsin hydrolyzes proteins at pH 3.2-3.5. Rennin (chymosin) causes milk to curdle in the presence of calcium ions, as it converts the soluble protein caseinogen into an insoluble form - casein. Gastric juice also contains non-proteolytic enzymes. Gastric lipase is little active and breaks down only emulsified fats. Hydrolysis of carbohydrates continues in the stomach under the influence of salivary enzymes. This becomes possible because the bolus of food that has entered the stomach is gradually saturated with acidic gastric juice, and at this time the action of salivary enzymes continues in the inner layers of the bolus of food in an alkaline environment. The composition of organic substances includes lysozyme, which provides the bactericidal properties of gastric juice. Gastric mucus containing mucin protects the gastric mucosa from mechanical and chemical irritation and from self-digestion. The stomach produces gastromucoprotein, or intrinsic Castle factor. Only in the presence of an internal factor is it possible to form a complex with vitamin B12, which is involved in erythropoiesis. Gastric juice also contains amino acids, urea, and uric acid.
Zones of acid production and neutralization in the stomach[edit | edit code]
The gastric stage of food digestion occurs with the help of enzymes, the most important of which is pepsin, which necessarily require an acidic environment. However, the acid in chyme (gruel), consisting of partially digested food and gastric juices, must be neutralized before being evacuated from the stomach.
The stomach can be conditionally divided into acid-forming (upper) and acid-neutralizing (lower) zones, separated by an intermediate zone, that is, a zone of transition from slightly acidic pH (6.0-4.0) to strongly acidic (pH less than 3.0) and located between body of the stomach and its antrum.
Since when studying the acidity of the stomach, information about the processes of acid production and acid neutralization is diagnostically important, the measurement of stomach acidity should take place in at least two zones: the body of the stomach and the antrum.
Neutralization of acid in the stomach is carried out mainly due to bicarbonate ions (HCO3-) secreted by the surface cells of the mucous membrane.
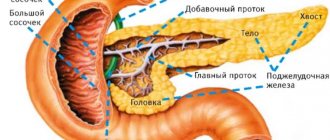
Composition and properties of pancreatic juice
The exocrine activity of the pancreas consists of the formation and secretion of 1.5-2.0 liters of pancreatic juice into the duodenum. The composition of pancreatic juice includes water and dry residue (0.12%), which is represented by inorganic and organic substances. The juice contains cations Na +, Ca 2+, K +, Mg + and anions Cl -, SO3 2-, HPO4 2-. It contains especially a lot of bicarbonates, due to which the pH of the juice is 7.8-8.5. Pancreatic juice enzymes are active in a slightly alkaline environment. Pancreatic juice is represented by proteolytic, lipolytic and amylolytic enzymes that break down proteins, fats, carbohydrates and nucleic acids. Alpha-amylase, lipase and nuclease are secreted in an active state; proteases - in the form of proenzymes. Pancreatic alpha-amylase breaks down polysaccharides into oligo-, di- and monosaccharides. Nucleic acids are broken down by ribo- and deoxyribonucleases. Pancreatic lipase, active in the presence of bile salts, acts on lipids, breaking them down into monoglycerides and fatty acids. Phospholipase A and esterase also act on lipids. In the presence of calcium ions, fat hydrolysis increases. Proteolytic enzymes are secreted in the form of proenzymes - trypsinogen, chymotrypsinogen, procarboxypeptidase A and B, proelastase. Under the influence of duodenal enterokinase, trypsinogen is converted into trypsin. Then trypsin itself acts autocatalytically on the remaining amount of trypsinogen and on other propeptidases, converting them into active enzymes. Trypsin, chymotrypsin, and elastase break down mainly the internal peptide bonds of food proteins, resulting in the formation of low molecular weight peptides and amino acids. Carboxypeptidases A and B cleave C-terminal bonds in proteins and peptides.
Three phases of hydrochloric acid secretion[edit | edit code]
- The secretion of hydrochloric acid begins even before food enters the stomach. The first phase of secretion (the so-called cephalic) is triggered by the smell, sight and taste of food, the effect of which is transmitted from the central nervous system to the cells of the stomach through the nerve endings innervating the stomach.
- The most significant phase of secretion is gastric, which begins after food enters the stomach. Distension of the stomach triggers the release of gastrin from G cells located in the antrum of the stomach. Gastrin, acting on parietal cells directly or through activation of ECL cells with the release of histamine, stimulates the production of hydrochloric acid.
- The final phase of secretion - intestinal - is triggered when food enters the duodenum and stretches it.
An increase in the acidity of gastric juice turns on a mechanism for regulating secretion: in the cells of the antrum of the stomach, the production of somatostatin, a blocker of hydrochloric acid secretion, is triggered.
Natural gastric juice
The composition of the drug includes digestive juice, as well as an alcohol solution of salicylic acid. The drug is used to normalize the level of acidity in the stomach and improve digestion processes. Natural gastric juice improves appetite and eliminates dyspeptic disorders. Experts prescribe the remedy for achylia, hypoacid and anacid gastritis.
Natural gastric has some limitations; it cannot be used in the following cases:
- gastroesophageal reflux;
- hyperacid gastritis;
- stomach and duodenal ulcers;
- erosive gastritis and duodenitis;
- allergy to active ingredients.
Proper storage of the drug plays an important role. If you leave the product in a warm place, it will lose its activity.
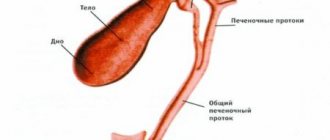
Hydrochloric acid is the main component of gastric juice
The most important component of gastric juice, which is produced by the parietal cells of the fundic glands of the stomach, is hydrochloric acid.
It maintains a certain level of acidity in the stomach, prevents pathogens from entering the body and prepares food for effective hydrolysis. Hydrochloric acid has a constant and unchanged concentration - 160 mmol/l.
Digestion begins in the mouth. Salivary enzymes - maltase and amylase - are involved in the breakdown of polysaccharides. The bolus of food enters the stomach, where approximately 30-40% of carbohydrates are digested with the help of gastric juice; as a result of exposure to hydrochloric acid, the alkaline environment changes to acidic, maltase and amylase are inactivated.