Helicobacter pylori is a pathogenic bacterium that lives mainly in the pylorus (antrum) of the stomach.
The photo below shows that the microorganism has the shape of a spiral to which flagella are attached. This structure helps it hold tightly to the walls of the digestive organ, move along it along with mucus and exist in an acidic environment, which many pathogenic microorganisms cannot tolerate and die.
Once in the human body, Helicobacter pylori causes a dangerous disease - Helicobacteriosis. Bacteria multiply rapidly, and in the process of their vital activity they produce a lot of toxins that corrode the mucous membrane of the stomach (duodenum), and then the walls of the digestive organ themselves. Such exposure is dangerous because it creates a favorable environment for gastritis, ulcers, and malignant neoplasms.
What it is?
Helicobacter pylori is just a bacterium that is found in patients with various diseases of the stomach and intestines, the duodenum in particular.
As for the name of the bacterium Helicobacter pylori, it is not at all accidental. One part of it, “pylori”, indicates the main habitat of the bacterium - the pyloric section of the stomach, and the second part, “heliko”, characterizes the shape of the bacterium: helical, spiral.
Previously in medicine it was believed that a microorganism capable of surviving in the acidic, salty environment of the stomach did not exist in principle. But then doctors did not suspect the existence of Helicobacter. Helicobacter pylori was discovered only in 1979 by Australian scientist Robin Warren. Together with scientific colleague Dr. Barry Marshall, the “discoverers” managed to grow this Helicobacter bacterium in laboratory conditions. Then they only assumed that it was she who was the culprit of gastritis and stomach ulcers, and not unhealthy diet or stress, as previously thought.
In an attempt to confirm the correctness of his guess, Barry Marshall conducted an experiment on himself by drinking the contents of a Petri dish in which Helicobacter pylori was cultivated. Just a few days later, the scientist was diagnosed with gastritis. He was cured by taking metronidazole for two weeks. And already in 2005, the authors of this discovery, scientists, received the Nobel Prize in the field of medicine for their discovery. The whole world has recognized that ulcers and gastritis, with all the resulting and associated diseases, appear precisely because of Helicobacter.
Discovery of Helicobacter pylori
The history of one of the greatest discoveries of the 20th century began in 1979, when ordinary doctor Barry Marshall received a position at the largest clinic in Western Australia - the Royal Perth Hospital. During one of the internal trainings organized by the clinic's management, Marshall met the talented pathologist Robin Warren. He was just studying the etiology of gastritis and managed to captivate his new friend. Already in 1981, the duo of Marshall and Warren discovered a spiral bacterium in the mucous membrane of patients with gastritis. It took researchers a year to develop a hypothesis linking the “newborn” Helicobacter pylori with strong ties to peptic ulcers and stomach cancer.
However, the road to success is rarely paved with roses and lilies. At first, the theory of Marshall and Warren met only grins from colleagues and skepticism from venerable scientists. In 1998, when the difficulties were over, Marshall wrote: “Everyone was against me then. But I knew I was right." Persistent Australians paved the way for their discovery, sparing neither time, nor strength, nor health.
After an unsuccessful attempt to infect piglets with Helicobacter pylori infection, Marshall decided on an experiment worthy of great researchers. He drank the contents of the Petri dish in which H. pylori was cultured and prepared to wait. The scientist hoped that in a year or a little more he would be diagnosed with a stomach ulcer, and he would finally prove an obvious cause-and-effect relationship. However, events were not long in coming.
Three days into the experiment, Marshall's mother noticed that her son's breath began to smell bad. Soon nausea and vomiting joined the symptoms, and just eight days after the desperate act, an endoscopic examination confirmed: acute gastritis had begun in the doctor’s stomach, and H. pylori was cultured from a smear of the mucous membrane. On the 14th day of the experiment, Marshall began taking antibiotics. After the course of treatment, complete recovery was recorded.
In 1985, details of the experiment appeared in the pages of the Medical Journal of Australia. By the way, this article soon gained worldwide fame and became the most cited in the entire history of the publication. It would seem that the evidence is obvious and the matter is small. However, full recognition was still almost a decade away.
Table 2.
Drugs for eradication therapy (international and trade names)
| Group | International name | Tradename |
| antibiotics penicillins | amoxicillin | Ospamox, Flemoxin Solutab, Hiconcil |
| macrolide antibiotics | clarithromycin | Labax, Klabax OD, Clarithromycin-Zentiva, Clarithromycin Pfizer, Klacid, Klacid SR, Fromilid, Fromilid Uno |
| antibiotics tetracyclines | tetracycline | Tetracycline |
| proton pump inhibitors | omeprazole | Zerotsid, Losek, Omez, Omizak, Ortanol, Romesek, Ulkozol, Ultop, Helitsid, Cisagast |
| lansoprazole | Lanzabel, Lanzap, Lanzoptol, Lancid, Loenzar-sanovel, Epicurus | |
| rabeprazole | Bereta, Zolispan, Zulbex, Noflux, Ontime, Pariet, Rabelok, Hayrabezol | |
| pantoprazole | Zipantola, Controloc, Crosacid, Nolpaza, Pantaz, Panum, Peptazol, Pigenum-sanovel, Puloref, Sanpraz, Ulthera | |
| esomeprazole | Nexium, Neo-Zext, Emanera | |
| antimicrobial and antiprotozoal agents | metronidazole | Klion, Metrogyl, Metronidazole, Trichopolum, Flagyl, Efloran |
| gastroprotectors | bismuth tripotassium dicitrate | De-Nol, Novobismol |
How can you get infected?
Infection occurs when bacteria are transmitted from one person to another through the fecal-oral or oral-oral route. In addition, there are hypotheses about the transmission of this bacteria from cats to humans, as well as about its mechanical transfer by flies.
Most often, infection occurs in childhood. The most likely route of infection is transmission of Helicobacter pylori from person to person, which can occur in three ways:
- Iatrogenic (caused by medical procedures) pathway. In this case, infection is caused by the use of an endoscopic or other medical instrument that came into contact with the gastric mucosa of an infected patient in another person.
- Fecal-oral route. H. pylori is shed in the stool of infected people. The source of infection can be water or food contaminated with feces.
- Oral-oral route. There is evidence that Helicobacter pylori can be present in the oral cavity. Therefore, transmission of bacteria is possible through sharing cutlery and toothbrushes, or kissing.
Publications
In gastroenterological practice, diseases of the stomach and duodenum in children are the most common. Helicobacter pylori (HP) infection plays a significant role in the development of inflammatory changes in the mucous membrane.
HP infection occurs mainly in childhood, and without appropriate treatment, the persistence of bacteria remains lifelong. According to a number of authors, the infection rate of HP in children over 7 years of age with abdominal pain and/or dyspeptic syndrome reaches 80%.
Depending on the virulent properties of the microorganism and the genetic characteristics of the macroorganism, the outcome of H. pylori infection may be different. Long-term persistence of the microorganism can lead to the development of atrophic multifocal gastritis, gastric adenocarcinoma, and MALT lymphoma. In recent years, there has been a tendency towards an increase in cases of severe changes in the stomach, leading to the development of serious complications, which significantly worsens the general condition of the child and requires long-term and expensive treatment.
The standards for the eradication of Helicobacter pylori infection, adopted by the 3rd Maastricht Consensus in 2005, refer to the necessary measures for a proactive response to the increase in H. pylori resistance to previously recommended regimens. According to modern regulations, a 14-day duration of treatment is more effective compared to a 7-day duration. In cases where high-quality “local” studies have demonstrated its effectiveness, a 7-day protocol can be used, which includes a proton pump inhibitor (PPI) at a standard dosage 2 times a day + clarithromycin 500 mg 2 times a day + amoxicillin 1000 mg 2 times per day (or metronidazole 500 mg 2 times). In accordance with modern regulations, treatment of HP infection should be economically affordable for the patient, well tolerated, and the number of cured patients should be at least 80%.
A combination drug registered in the Republic of Belarus that complies with the recommendations of EHPSG - 2005 is Peptipac . One package contains seven blisters, each of which contains a daily set of drugs for 1st line eradication: omeprazole (Omepral) 20 mg × 2 times, clarithromycin (Claricar) 500 mg × 2 times, amoxicillin (Amoxicar) 1000 mg × 2 times a day. All medications are taken 20-30 minutes before meals. Convenience in distributing the daily dose allows the patient and his parents to adhere to therapy.
First-line therapy is recommended if the resistance of the most common strains of H. pylori to clarithromycin in a given region does not exceed 10-15%, and to metronidazole - 40%. In first-line therapy, Maastricht-3 suggests the possibility of replacing amoxicillin with metronidazole (500 mg × 2 times), if the resistance of the most common HP strains to metronidazole does not exceed 40%. In our region, the frequency of antibiotic resistance of HP strains to metronidazole ranges from 40% to 55.5%, so the use of triple eradication protocols that include this drug is not effective enough.
Recently, to increase the effectiveness of eradication therapy, regimens have been proposed that include bismuth tripotassium dicitrate. Quad therapy using bismuth-containing drugs is a possible alternative as a “first choice” protocol. The last provision is new from the point of view of normative statements, although it has been used in practice previously.
According to the Maastricht-3 recommendations, eradication of HP in children should be carried out with the same drugs as in adults, with daily doses of drugs calculated based on body weight. Studies conducted in our country in adults have shown that the protocol of choice for eradication therapy is a 7-day triple therapy (PPI-clarithromycin-amoxicillin). The high frequency of Helicobacter pylori infection in the pediatric population necessitates the selection of eradication regimens and duration of treatment that are optimal specifically for childhood.
The purpose of this study was to determine the effectiveness of a first-line protocol for the treatment of Helicobacter pylori infection in children (PeptiPac) (omeprazole-clarithromycin-amoxicillin) and to evaluate the effectiveness of this regimen supplemented with tripotassium bismuth dicitrate.
Patients and research methods
We observed 38 patients (22 boys and 16 girls) aged 10 to 17 years with a diagnosis of chronic gastritis associated with Helicobacter pylori infection. The diagnosis was established on the basis of anamnesis, characteristic complaints, objective examination data, laboratory, endoscopic and morphological studies. Criteria for inclusion in the study: availability of data from morphological examination of gastrobiopsy specimens; the patient did not receive antisecretory, antibacterial or antacid drugs; voluntary informed consent of parents for their child’s participation in the study. Exclusion criteria from the study: duodenal ulcer, intolerance to penicillin antibiotics or macrolides.
All patients received treatment at the 17th Children's City Clinical Clinic. Esophagogastroduodenoscopy (EGDS) with targeted biopsy of the mucous membrane (SM) of the body and antrum of the stomach was carried out on the basis of the 2nd DKB, 4th DKB and DIKB in all patients before the start of eradication therapy. A control endoscopy with biopsy was performed 4-8 weeks after the end of treatment (but not earlier than 2 weeks after discontinuation of the PPI). H. pylori was detected by the bacterioscopic method in gastrobiopsy specimens, and during control, also by the rapid urease method. The success of eradication was stated in the absence of HP in biopsy specimens and a negative result of the rapid urease test.
When assessing the effectiveness of treatment, the percentage of patients cured of HP infection in each group was calculated. Four children who received eradication therapy dropped out of further study due to refusal of repeat endoscopy due to lack of complaints (3 people from group I and 1 from group II).
The patients were divided into two groups. Children with verified HP infection received one of two anti-Helicobacter therapy regimens (Table 1).
Table 1. First-line eradication therapy protocols
| Group number | Number of patients | Eradication protocol | Duration of therapy (days) |
| I | 22 | omeprazole, amoxicillin, clarithromycin (UAC-7) | 7 |
| II | 16 | bismuth tripotassium dicitrate, omeprazole, amoxicillin, clarithromycin (BOAC-7) | 7 |
Research results and discussion
Hereditary burden of peptic ulcer and stomach cancer was detected in 10 children (26.3%). The duration of symptoms of gastric dyspepsia before inclusion in the study ranged from 6 months. up to 3 years. During examination, patients most often complained of pain in the epigastric region, occurring both before and after meals, early satiety, loss of appetite, nausea, belching, bloating, and unstable stool. The clinical picture was dominated by a mixed variant of functional dyspepsia (in 20 children); dyskinetic (15 patients) and ulcer-like (3 people) variants were less common. Pain on palpation in the epigastrium and pyloroduodenal area was observed in almost all children.
EGD revealed inflammatory changes in the gastric and duodenal mucosa (edema, hyperemia, hypersecretion) of varying severity in 37 patients; no endoscopic changes were found in 1 child. Defects of the mucous membrane in the form of single or multiple erosions were noted in eight cases. Concomitant motility disorders of the upper digestive tract: gastroesophageal reflux disease with grade I-II esophagitis was detected in 8 children (21%), of which duodenogastric reflux was noted in 2 cases.
Histological examination of biopsy samples of the gastric mucosa revealed pangastritis in 26 subjects (68.4%). Isolated damage to the antrum was observed in 29% of cases (11 patients), and to the body of the stomach - in 1 patient. According to bacterioscopy, degree I contamination of the antrum mucosa with HP was detected in 7 patients (18.4%), degree II - in 14 (36.8%) and degree III - in 17 children (44.8%). According to bacterioscopy and the results of a rapid urease test, eradication was achieved in 16 (84.4%) patients in group I, and in 14 (93.3%) patients in group II (Table 2).
Table 2. Results of eradication therapy
| Group number | Number of patients | Efficiency of eradication of abs. (%) | |
| HP (-) | HP (+) | ||
| I | 19 | 16 (84,4%) | 3 (15,6%) |
| II | 15 | 14 (93,3%) | 1 (6,7%) |
There were no significant side effects that led to cessation of treatment in the children we observed. There were no cases of skin rash or other allergic manifestations to the medications. Two patients reported an increase in stool frequency up to 4 times per day for 2 days, three reported nausea and two reported a metallic taste in the mouth. The number of side effects was the same in both groups.
Histological studies of biopsy specimens after eradication therapy showed that after a month in most patients, neutrophilic and mononuclear infiltration of the epithelium and lamina propria of the mucous membrane disappeared, and the activity and intensity of inflammation of the gastric mucosa significantly decreased (Table 3).
Table 3. Histological indicators of the activity and severity of inflammation in the gastric mucosa before and after eradication
| Histological indicators | Stomach mucosa | |||
| fundus before treatment (n=38) / after treatment (n=34) | antrum before treatment (n=38) / after treatment (n=34) | |||
| abs. | % | abs. | % | |
| Inflammatory activity | ||||
| — | 11/19 | 28,9/55,9 | 8/16 | 21,1/47,1 |
| + | 16/12 | 42,1/35,3 | 11/13 | 28,9/38 |
| ++ | 9/3 | 23,7/8,8 | 16/4 | 42/11,7 |
| +++ | 2/- | 5,3/- | 3/1 | 8/3,2 |
| Intensity of inflammation | ||||
| — | 15/22 | 39,5/64,7 | 1/19 | 2,6/55,9 |
| + | 12/10 | 31,6/29.4 | 4/12 | 10,5/35,3 |
| ++ | 7/2 | 18,4/5,9 | 23/3 | 60,5/8,8 |
| +++ | 4/- | 10,5/- | 10/- | 26,4/- |
conclusions
1. Seven-day first-line triple therapy ( Peptipak ) in children with chronic HP-associated acid-related diseases provides a high percentage (84.4%) of eradication and improves clinical, endoscopic and morphological parameters.
2. With a slight increase in the cost of treatment, the effectiveness of eradication increases (p < 0.01) when bismuth tripotassium dicitrate is included in the regimen.
3. Peptipak is well tolerated; no clinically significant adverse reactions were observed in the studied groups of children.
Literature:
1. Aruin L.I. Quality of healing of gastroduodenal ulcers: functional morphology, the role of pathogenetic therapy methods // Experimental and clinical gastroenterology. Reprint. 2006. 5p. 2. Bovbel I.E. Comparative analysis of clinical and morphological changes and indicators of lipid metabolism, the level of medium molecular peptides in children with chronic gastroduodenitis. Author's abstract. diss., cand. honey. Sci. Minsk, 1999. – 22 p. 3. Diseases of the esophagus and stomach. Ivashkin V.T., Sheptulin A.A. Brief practical guide. – M.: MEDpress-inform, 2002. – 144 p. 4. Isakov V.A. Therapy of acid-dependent diseases with proton pump inhibitors in questions and answers // Gastroenterology / surgery. Reprint.2006, 7 p. 5. Lapina T.L. Macrolide antibiotic clarithromycin in eradication therapy of Helicobacter pylori infection // Breast Cancer. Application. Diseases of the digestive system. Volume 8, No. 1, 2006. P.39-42. 6. Loginov A.F. "Maastricht-3" - modern tactics for diagnosing and treating Helicobacter pylori infection // Farmateka. No. 12 (127) 2006. - P.46-48. 7. Maev I.V., Samsonov A.A. Modern standards of treatment of acid-related diseases associated with H. pylori (materials of the Maastricht-3 consensus) // Gastroenterology. Supplement to the journal Consilicum medicum No. 1, 2006.P.3-8 8. Pimanov S.I., Makarenko E.V. Analysis of the effectiveness of Helicobacter pylori infection eradication protocols // Recipe. – 2005. – No. 1. – P. 19-23. 9. Pimanov S.I., Makarenko E.V., Koroleva Yu.I. Possibilities of empirical eradication therapy in patients with duodenal ulcer in the Republic of Belarus // Recipe. – 2006. – No. 1 (45). – P. 56-60. 10. Khavkin A.I., Zhikhareva N.S. Modern principles of anti-Helicobacter therapy in children // Russian Medical Journal. Volume 13, No. 3, 2005. P.137-139.
I.E.Bovbel, V.Yu. Malyugin
“Medical Panorama” 2008, No. 3 art. 57-58
What happens in the body?
At the initial stage, after entering the stomach, H. pylori, quickly moving with the help of flagella, overcomes the protective layer of mucus and colonizes the gastric mucosa. Having established itself on the surface of the mucous membrane, the bacterium begins to produce urease, due to which the concentration of ammonia increases in the mucous membrane and the layer of protective mucus near the growing colony and the pH rises. By a negative feedback mechanism, this causes an increase in the secretion of gastrin by the cells of the gastric mucosa and a compensatory increase in the secretion of hydrochloric acid and pepsin, with a simultaneous decrease in the secretion of bicarbonates.
Mucinase, protease and lipase produced by the bacterium cause depolymerization and dissolution of the protective stomach mucus, as a result of which hydrochloric acid and pepsin gain direct access to the exposed gastric mucosa and begin to corrode it, causing a chemical burn, inflammation and ulceration of the mucous membrane.
The endotoxin VacA produced by the bacterium causes vacuolation and death of gastric epithelial cells. Products of the cagA gene cause degeneration of gastric epithelial cells, causing changes in cell phenotype (cells become elongated, acquiring the so-called “hummingbird phenotype”). Attracted by inflammation (in particular, the secretion of interleukin-8 by cells of the gastric mucosa), leukocytes produce various inflammatory mediators, which leads to the progression of inflammation and ulceration of the mucosa; the bacterium also causes oxidative stress and triggers the mechanism of programmed cell death of gastric epithelial cells.
Antibacterial drugs in treatment regimens for helicobacteriosis in children
Studies conducted in many countries around the world have confirmed that the properties of H. pylori correspond to Koch’s postulates, which determine the infectious agent of a particular disease. Moreover, a direct relationship between the effect of H. pylori on the gastric mucosa and the occurrence of gastric malignancies has now been proven. Throughout the history of studying H. pylori infection, scientists and researchers in many countries have developed various approaches to the treatment of inflammatory diseases of the upper digestive tract associated with this microorganism. The effectiveness of using both a single-component antibiotic treatment regimen and a combination of drugs from different groups was studied. As a result of these studies, it was shown that some antibacterial drugs are not able to exert their effect in the living conditions of H. pylori (in the acidic environment of the stomach), for the effective use of others, patients needed to take very large doses of the drug, and when using others, resistance of the microorganism quickly developed to the medications used. However, to date, treatment regimens have been developed that can effectively combat H. pylori, achieving its complete eradication (destruction) in a short time, and achieving long-term remission of the disease. For effective treatment of gastroenterological diseases associated with Helicobacter pylori infection, the use of specific antibacterial drugs is necessary. Considering the ecological niche that these microorganisms occupy, the antibacterial therapy carried out must meet certain requirements, namely: 1. The drugs used must effectively act on H. pylori; 2. Antibiotics must be resistant to the aggressive acidic environment of the stomach; 3. They must have the ability to penetrate under the layer of gastric mucus; 4. Have a local effect in the mucous membrane area; 5. Quickly eliminated from the body without accumulating in other tissues and organs. Helicobacter, being microaerophiles, is able to function normally only in its usual habitat - on the surface of the mucous membrane under a layer of mucus. Outside the body, they are extremely sensitive to almost any aggressive influence (alcohol, atmospheric air, antibacterial drugs acting on gram-negative flora). However, gastric mucus, changes in the pH of the environment in the lumen of the stomach and in the immediate vicinity of H. pylori largely change the effect of drugs. These conditions significantly narrow the range of drugs used to treat helicobacteriosis. The problem of treating helicobacteriosis in adults, according to some authors, is solved by prescribing a short course (3–4 days) of a combination of antibiotics within the maximum permitted doses [1,2,3,4,5], which, however, is unacceptable in pediatrics. At the same time, in children with gastroduodenal pathology caused by the presence of Helicobacter, changes in the biocenosis of the gastrointestinal tract appear. Thus, a number of authors identified a significant deficiency of lactobacilli and bifid flora with a simultaneous increase in the level of opportunistic microorganisms against the background of chronic gastroduodenal diseases; the severity of the disorders increased during antibacterial therapy [3,6]. At the end of the 80s, at the dawn of the “era of helicobacteriosis,” it was believed that for the effective treatment of chronic inflammatory diseases of the ascending digestive tract, it was sufficient to prescribe one drug (monotherapy) or dual therapy. For these purposes, metronidazole was usually used in combination with colloidal bismuth subcitrate or semisynthetic penicillin antibiotics in age-related dosages. Treatment lasted an average of 14–28 days and resulted in eradication in 70–80% of children. However, a long course of treatment, widespread and not always justified prescription of antibiotics for gastroduodenitis and peptic ulcers in children and adults led to the fact that by 1991 the use of one or two drugs did not cause eradication. For effective treatment of helicobacteriosis, other antibiotics were included in the treatment regimen. It should be noted that the use of colloidal bismuth subcitrate slows down the absorption of some antibacterial drugs (tetracycline, clarithromycin, amoxicillin), thereby increasing their concentration in the gastric contents - the site of application in the treatment of helicobacteriosis. In 1992–94 The most effective was a triple treatment regimen, including colloidal bismuth subcitrate 120 mg 3 times a day, metronidazole 20 mg/kg/day in 3 doses, amoxicillin 30 mg/kg 3 times a day. However, already from mid-1994, the degree of eradication from the therapy decreased and amounted to only 46.4%, and reinfection in previously cured children occurred after 1–4 months [3,5,8]. Given the increasing number of strains resistant to metronidazole, research has been actively conducted using new effective antibacterial drugs that can achieve good results in eradication therapy of H. pylori. As a result of these studies, a number of provisions were formulated, presented at a meeting of the H. pylori Working Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHN) in October 2000. In particular, these recommendations give preference to the use of eradication three-component regimens combining proton pump inhibitors and two antibiotics, one of which is clarithromycin.In accordance with these recommendations at the IX Congress of Pediatricians of Russia "Children's Healthcare of Russia: Development Strategy" (February 2000, Moscow) treatment regimens for helicobacteriosis in children were adopted and approved. The drugs recommended for use in children from 5 years of age in eradication therapy include the following: Various combinations of these drugs made it possible to create the following treatment regimens for helicobacteriosis: I One-week triple therapy with a bismuth drug : 1. – Bismuth subcitrate; – Amoxicillin or clarithromycin; – Nifuratel/furazolidone. 2. – Bismuth subcitrate; – Clarithromycin; - Amoxicillin. II One-week triple therapy with H+, K+–ATPase blockers: 1. – Omeprazole; – Clarithromycin; – Nifuratel/furazolidone. 2. – Omeprazole; – Clarithromycin; - Amoxicillin. III One-week quad therapy: – Bismuth subcitrate; – Amoxicillin or clarithromycin; – Nifuratel/furazolidone; – Omeprazole/ranitidine. Quad therapy is recommended for the treatment of antibiotic-resistant strains when previous treatment has failed or when determining the sensitivity of the microorganism strain is not possible. The main drug for the treatment of H. pylori infection in children is colloidal bismuth subcitrate, which is combined with antibiotics - amoxicillin or various macrolides. Unfortunately, the regimens that were popular until recently, combining bismuth subcitrate, an antibiotic and metronidazole, have begun to lose their effectiveness in recent years. This is due to the emergence of drug-resistant strains. H. pylori does not develop resistance to bismuth preparations, and there is practically no resistance to amoxicillin (only a few strains have been described, including 2 in Russia, which are not sensitive to this antibiotic). At the same time, the number of strains resistant to metronidazole and clarithromycin is constantly increasing (and also, given the cross-resistance of H. pylori, to other macrolides). Currently, the number of resistant strains in children to metronidazole exceeds 42%, and to clarithromycin - 12%. Strains with combined resistance to metronidazole and antibiotics have appeared. That is why much attention has recently been paid to the development of new alternative treatment regimens for helicobacteriosis. Metronidazole is excluded from existing regimens, and instead Macmiror and furazolidone are increasingly used. These drugs were first used in gynecology to treat strains of chlamydia resistant to metronidazole. Quite a lot of work has been devoted to studying the effectiveness of furazolidone against H. pylori as part of eradication regimens. Furazolidone is an effective drug to which H. pylori develops virtually no resistance. However, the use of this drug has a number of limitations. In particular, in a number of countries (Japan, Korea, Lebanon, Italy), the use of furazolidone was prohibited or severely limited due to possible mutagenic properties that were detected in animal studies. However, WHO allows the use of this drug in short courses. Other significant disadvantages of furazolidone are unsatisfactory organoleptic properties (when taking this drug, many children complain of bitterness in the mouth and nausea). In addition, to achieve optimal concentrations of the drug in the body, it must be taken four times a day, unlike other components of eradication therapy. These qualities of furazolidone significantly reduce the compliance of the entire treatment regimen and thereby the effectiveness of the eradication. Another drug from the nitrofuran group is Macmiror (nifuratel). This drug is similar in its action to furazolidone, but unlike the latter it is safe even with long-term use. In addition, it has good organoleptic properties and does not cause discomfort, bitterness or nausea when taken. The half-life of the drug is long enough, which makes it possible to use Macmiror 2 times a day, like the other components of anti-Helicobacter therapy. Quite a large number of studies have already been carried out on the use of eradication regimens with Macmiror as a second antibiotic [9,10,11]. Currently, Macmiror is included in the recommendations of the Union of Pediatricians of Russia for the diagnosis and treatment of diseases associated with helicobacteriosis in children. As the results of our own studies have shown, the effectiveness of treatment regimens using Macmiror in combination with bismuth subcitrate and amoxicillin is more than 84%. Amoxicillin is a highly effective drug; resistance to H. pylori practically does not develop. However, the use of this antibiotic is limited, on the one hand, by its pronounced effect on the intestinal microflora. On the other hand, the use of penicillin antibiotics is limited by the high incidence of allergic reactions. That is why macrolides remain the antibiotics of choice in the treatment of H. pylori infection. In the group of macrolides for the treatment of diseases associated with H. pylori, clarithromycin is recommended as a first-line drug. This drug is resistant to the action of hydrochloric acid of the stomach and is quickly absorbed in the gastrointestinal tract regardless of food intake. The bioavailability of clarithromycin in children when prescribed at a dose of 7.5 mg/kg is 52–55%. Peak plasma concentrations and PFC (area under the pharmacokinetic curve) increase when taking the drug with food by 28 and 42%, respectively [12]. The greatest effect from the use of macrolides was shown by regimens combining proton pump inhibitors and macrolides. The effectiveness of clarithromycin in eradication regimens for helicobacteriosis is due to the favorable pharmacokinetic interaction when clarithromycin is combined with proton pump inhibitors. Clarithromycin inhibits the metabolism of omeprazole, and omeprazole, in turn, inhibits the metabolism of clarithromycin, resulting in an increase in the concentrations of both drugs in the blood plasma and the occurrence of a cumulative effect when they are used [13]. A limitation of the use of antibiotics in treatment regimens for diseases associated with H. pylori is the development of resistance of these microorganisms to the drugs used. To date, the sensitivity of H. pylori to clarithromycin in a number of Western European countries is 83–89%, but this figure is decreasing. This is due, on the one hand, to the unjustified prescription of macrolides in the treatment of various infectious diseases, and on the other hand, to the development of point mutations of 23S ribosomal RNA in H. pylori during previous eradication measures that did not lead to the complete destruction of the microorganism. However, despite the increasing number of strains resistant to clarithromycin, eradication regimens based on this antibiotic in combination with omeprazole remain leading in various studies to this day and allow achieving eradication in 82–88% of patients [14]. Thus, the effectiveness of eradication therapy for diseases associated with H. pylori infection largely depends on competently administered antibiotic therapy in eradication regimens, taking into account both the pharmacokinetic effect of the drugs and the socio-economic aspects of the treatment.

References 1. Atherton JC; Hudson N; Kirk G.E.; et al. Amoxycillin capsules with omeprazole for the eradication of Helicobacter pylori. Assessment of the importance of antibiotic dose timing in relation to meals.//AU: // Aliment Pharmacol Ther.– 1994.– Oct. 8(5).– P. 495–8.. 2. Sobhani I; Chastang C; De Korwin JD; et al. Antibiotic versus maintenance therapy in the prevention of duodenal ulcer recurrence. Results of a multicentric double–blind randomized trial./ // Gastroenterol Clin Biol. – 1995. – Mar. –19(3).– P. 252–8. 3. Sieber CC; Frei R; Beglinger C; et al. Helicobacter pylori resistance against metronidazole in Switzerland: implications for eradication therapy? // Schweiz Med Wochenschr.– 1994.– Aug 9. 124(31–32).– P.1381–4. 4. Marshall BJ, Warren JR Unindencifield curved bacilli in the stomach of patient with gastritis and peptic ulceration. // Lancet.– 1984.– v.1.– p.1311. 5. O'Connor HJ. Eradication of Helicobacter pylori.// AADE Ed J. – 1994. – Dec. 6 Suppl 1P.– S113–9. 6. Lykova E.A., Sidorenko S.V., Bondarenko V.M. and others. Antibacterial therapy and correction of microecological disorders in helicobacteriosis in children. // Diagnostics and treatment.– 1996.– No. 12.– P. 75– 77 7. Zala G; Wirth HP; Bauer S; et al. Eradication of metronidazole–resistant Helicobacter pylori: is omeprazole/amoxicillin a therapeutic alternative? Schweiz Med Wochenschr.– 1994.– Aug 9. 124(31–32).– P. 1385–90. 8. Chronic gastritis / Aruin L.I., Grigoriev P.Ya., Isakov V.A., Yakovenko E.P. // Amsterdam. – 1993. – 362 p. 9. Lazutina T. A., Christ A. A. Efficiency of eradication therapy for duodenal ulcer // Russian Journal. gastroent and hepat. 2003, No. 3, Appendix 19, p. 28 10. Malyamova L.N., Glazyrina N.V., Yurchenko E.Yu./Comparative effectiveness of anti-Helicobacter therapy regimens in children with diseases of the gastroduodenal region.// Russian journal. gastroent and hepat. 2003, No. 3, Appendix 19, p. 48 11. Shcherbakov P.L., Vartapetova E.E., Salmova V.S. et al./Comparative assessment of the effectiveness and safety of bismuth preparations in children.// Russian journal. gastroent and hepat. 2003, No. 3, Appendix 19, p. 56–59 12. Gan VN, Chu SY et al. Pharmacokinetics of a clarithromycin suspension in infants and children. Antimicrob. Agents Chemother., 1992, 36^2478–2480. 13. Mainz D., Borner K., Koeppe P. et al. Parmacokinetics of lansoprazole, amoxicillin and clarithromycin after simultaneous and single administration. In: The 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Toronto, 1997: abstr. A.–120A. 14. Huang J.–Q., Hunt RH The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta–analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxycillin or metronidazole. Aliment. Pharmacol. Ther. 1999: 13: 719–729.
Misconceptions about Helicobacter pylori
Often, when Helicobacter pylori is detected, patients begin to worry about their eradication (destruction). The mere presence of Helicobacter pylori in the gastrointestinal tract is not a reason for immediate treatment with antibiotics or other agents. In Russia, the number of Helicobacter pylori carriers reaches 70% of the population and the vast majority of them do not suffer from any diseases of the gastrointestinal tract. The eradication procedure involves taking two antibiotics (for example, clarithromycin and amoxicillin).
In patients with hypersensitivity to antibiotics, allergic reactions are possible - from antibiotic-associated diarrhea (not a serious disease) to pseudomembranous colitis, the likelihood of which is low, but the percentage of deaths is high. In addition, taking antibiotics negatively affects the “friendly” microflora of the intestine and genitourinary tract and contributes to the development of resistance to this type of antibiotic. There is evidence that after successful eradication of Helicobacter pylori, reinfection of the gastric mucosa is most often observed over the next few years, which after 3 years is 32±11%, after 5 years - 82–87%, and after 7 years - 90.9% ( Zimmerman Ya.S.).
Until pain appears, helicobacteriosis should not be treated. Moreover, it is generally not recommended to carry out eradication therapy in children under eight years of age, because their immunity has not yet been formed and antibodies to Helicobacter pylori are not produced. If they are eradicated before the age of 8, then a day later, after briefly interacting with other children, they will “catch” these bacteria (P.L. Shcherbakov).
Helicobacter pylori clearly requires eradication if the patient has a stomach or duodenal ulcer, MALTOMA, or if he has had a gastric resection for cancer. Many reputable gastroenterologists (not all) also include atrophic gastritis in this list. Helicobacter pylori eradication may be recommended to reduce the risk of developing stomach cancer. It is known that at least 90% of cases of stomach cancer are associated with H. pylori infection (Starostin B.D.).
Peptic ulcer disease in the world of big numbers
According to various sources, from 10 to 15% of the world's population will experience this disease at some point in their lives. Both in Russia and throughout the world, peptic ulcer disease is considered the most common gastrointestinal pathology, and with age the likelihood of getting sick becomes higher. This is largely due to the fact that the risk of “catching” the culprit of all troubles - small, but very resistant in an acidic environment, Helicobacter pylori - only grows over the years. And if at the age of 20 the probability of infection is 20%, then with each subsequent decade it increases by exactly 10%. It turns out that by the beginning of their sixties, half of the planet’s inhabitants are already intimately familiar with H. pylori.
Every year more and more new cases of the disease are diagnosed. Doctors attribute this disappointing trend to the widespread use of non-steroidal anti-inflammatory drugs, which significantly increase the vulnerability of the stomach wall. However, the achievements of modern medicine make it possible to give a very favorable prognosis for peptic ulcer disease. But back at the end of the last century, everything was not so clear.
Table 1.
Eradication therapy regimens
| A drug | Dose | Frequency of application |
| 1st line eradication therapy (7–10 days) | ||
| IPP | standard dose | 2 times a day |
| clarithromycin | 500 mg | |
| amoxicillin | 1g | |
| 2nd line therapy (10–14 days) - if first line therapy is ineffective or intolerant | ||
| IPP | standard dose | 2 times a day |
| metronidazole or amoxicillin | 500 mg (amoxicillin - 1 g) | 3 times a day (amoxicillin 2 times a day) |
| tetracycline | 500 mg | 4 times a day |
| bismuth drug | 120 mg | |
Symptoms and first signs
The development of infection in the digestive tract for a long time is practically asymptomatic. Bacteria attach to the mucous membrane of the intestine and duodenum, producing a toxic enzyme that gradually eats away the cells of epithelial tissues.
Only when erosions and ulcers appear on the walls of the organ does the patient begin to be bothered by the unpleasant symptoms of Helicobacter pylori:
- feeling of bloating and fullness in the stomach after eating;
- frequent belching with an acidic taste in the mouth;
- stomach pain regularly;
- there is a burning sensation in the esophagus, a bitter taste in the mouth;
- regular attacks of nausea, vomiting;
- increased gas formation, which provokes colic and discomfort.
In adults, unpleasant signs of Helicobacter pylori bacteria appear most often after eating and do not go away even after bowel movements. The patient is overcome by lethargy, loss of strength, drowsiness, and irritability. The presence of helicobacter pylori in the stomach or duodenum may be accompanied by a small skin rash, particularly on the face. With gastritis or ulcers caused by Helicobacter pylori, the patient complains of changes in stool (constipation or diarrhea), bad breath, brittle nail plate and constant general malaise.
Maastricht recommendations and general requirements for therapy
The discovery that the leading etiological and pathogenetic factor in the development of inflammatory diseases of the lining of the stomach and duodenum is the pathogen Helicobacter pylori, marked the beginning of the development of fundamentally new therapeutic regimens. They were based on the use of antibiotics, which have the ability to directly destroy bacteria. Studying the structural features of the microorganism, understanding what Helicobacter is and how it has a negative effect on the mucous membrane, has made it possible to create effective treatment regimens that are successfully used today all over the world.
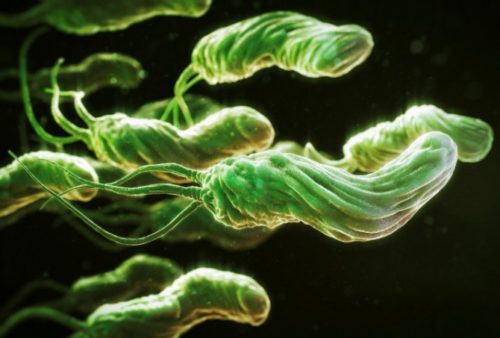
Helicobacter pylori is a spiral-shaped gram-negative bacterium
To identify Helicobacter, a urease breath test is used, which does not require much time, is harmless, does not cause any discomfort and is the gold standard for diagnosing infection. Symptoms and treatment of Helicobacter pylori infection vary from person to person. The presence of a pathogen in the body is not always accompanied by disturbances and requires taking medications.
To clarify and agree on the best approaches to identifying and treating Helicobacter in Europe, a group of leading gastroenterologists was formed. Periodically, based on conferences and meetings, they publish protocols - the Maastricht Consensus, named after the city where the first meetings took place.
There are currently five of them:
- Maastricht 1 (1996, Maastricht);
- Maastricht 2 (2000, Maastricht);
- Maastricht 3 (2005, Florence);
- Maastricht 4 (2012, Florence);
- Maastricht 5 (2016, Florence).
According to the results of the Maastricht 2 consensus, it was found that, unfortunately, none of the eradication regimens being carried out, despite the simultaneous use of several drugs, guarantees complete destruction of the infection. In this regard, it was recommended to treat patients first with a first-line regimen, and then, in the absence of a therapeutic effect, with a second-line regimen.
The need for repeated courses of anti-Helicobacter therapy is explained by the increased resistance of H. pylori to prescribed antibiotics, which is the main problem of modern gastroenterology in the treatment of diseases associated with the pathogen.
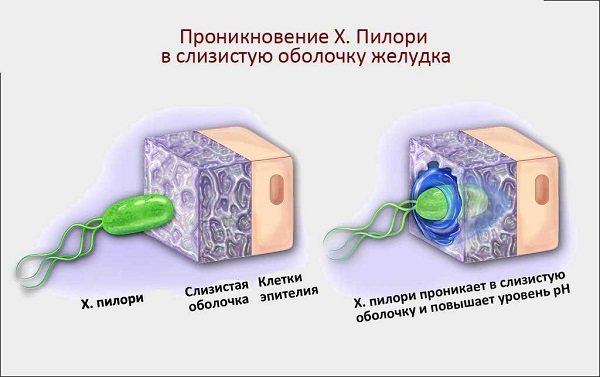
Penetration of the pathogen into the gastric mucosa
The eradication regimens used today to cure Helicobacter pylori infection were approved at Maastricht 4.
Basic requirements for them:
- obtaining at least 80% of cases of getting rid of infection, healing of gastritis or ulcers, confirmed by repeated research;
- duration of therapy is no more than 14 days;
- acceptable low toxicity of the drugs used;
- safety, side effects in less than 15% of patients;
- absence of serious reactions requiring early discontinuation of the pills;
- accessibility and convenience for patients, a small number of medication doses per day;
- overcoming the ever-increasing resistance of bacteria to antibiotics;
- the possibility of replacing drugs within the regimen if allergic reactions or other problems are identified.
Interesting! Historically, the first successfully tested Helicobacter eradication regimen was the use of bismuth subsalicylate and Metronidazole. It was tested by scientist Barry Marshall, who together with a colleague discovered Helicobacter pylori in the gastric mucosa. In 1984, he deliberately drank the contents of a Petri dish containing a culture of bacteria, and then, shortly after the appearance of characteristic symptoms of provoked gastritis, carried out the indicated treatment. After 14 days of therapy, according to the results of a biopsy, no pathogen was found in the stomach.
What diseases can be caused by H. pylori
The presence of H. pylori in the stomach is not a disease in itself. However, these bacteria increase the risk of developing various diseases of the digestive tract.
Although colonization of the gastric mucosa by Helicobacter causes histological gastritis in all infected individuals, only a small proportion of them develop clinical symptoms of this disease. Scientists estimate that 10-20% of people infected with Helicobacter pylori develop ulcers, and 1-2% develop stomach cancer.
Diseases the development of which is associated with Helicobacter pylori infection:
- Gastritis is an inflammation of the gastric mucosa. Soon after infection with H. pylori, a person develops acute gastritis, sometimes associated with dyspepsia or nausea. An acute inflammatory process affects the entire stomach and leads to a decrease in acid secretion. After a certain period of time after acute gastritis, chronic gastritis develops.
- Ulcer of the stomach and duodenum. According to scientific data, 70-85% of all stomach ulcers and 90-95% of all duodenal ulcers are caused by bacteria
- Functional dyspepsia is pain in the upper abdomen that is not caused by an ulcer or other stomach lesions. Scientific research has shown that some types of dyspepsia are associated with infection. Treatment to eradicate the bacteria provides relief for many patients with functional dyspepsia and also reduces the risk of developing stomach ulcers and cancer in the future.
- Stomach cancer. Helicobacter pylori is a scientifically recognized etiological factor in the development of stomach cancer. According to one hypothesis, bacteria promote the production of free radicals and increase the risk of mutations in stomach cells.
- MALT lymphoma of the stomach. The infection's association with this disease was first reported in 1991. This bacterium is believed to cause 92-98% of gastric MALT lymphomas.
The fight for recognition
It was not until 1994 that the US National Institutes of Health documented the close association between H. pylori and peptic ulcers and recommended treating it with antibiotics. However, in 1995, most patients with stomach ulcers were still receiving antisecretory therapy, and only 5% of the lucky ones met doctors who prescribed antibiotics.
In the same 1995, the American Health Foundation conducted a large survey among people suffering from peptic ulcers. The results were impressive. More than 10 years after the discovery of H. pylori, 90% of patients had no idea that the true cause of their illness was an infection and blamed stress and weak nerves.
In 1996, the American Drug Administration (FDA) approved the use of an antibiotic for the treatment of peptic ulcers for the first time in the world. A year later, in the same United States, a national campaign was launched, the purpose of which was to inform doctors and pharmacists about the connection between gastric ulcers and H. pylori. Soon the news of the absolute curability of the previously quite serious disease spread throughout countries, cities and villages, and a new era in the treatment of peptic ulcer disease began.
Table 3.
Combination drugs for eradication therapy
| International name | Tradename |
| omeprazole+amoxicillin+clarithromycin | Pilobact AM |
| lansoprazole+amoxicillin+clarithromycin | Lancid Keith, Helitrix |
What about the discoverers? They fully felt the sweet burden of glory and continue to bask in its rays to this day. In 2005, at the Karolinska Institute in Stockholm, Marshall and Robin William received the most prestigious prize, which has been awarded for more than 100 years for outstanding achievements in science and culture. The gold medal, accompanied by a diploma from the Nobel Committee and a very substantial monetary reward, was awarded to Australian scientists for “the discovery of the bacterium Helicobacter pylori and its role in the development of gastritis and peptic ulcers.” And eradication therapy, which began back in 1982, continues to save millions of patients, curing ulcers and preventing cancer.
Preparations for Helicobacter pylori eradication
Today, several regimens for Helicobacter pylori eradication therapy have been developed, which are selected individually.
The traditional anti-Helicobacter complex includes: an antibiotic (amoxicillin, clarithromycin or tetracycline); proton pump inhibitor; metronidazole; bismuth preparations.
Amoxicillin
The penicillin antibiotic, amoxicillin, is very close to ampicillin both structurally and in its spectrum of activity. Amoxicillin is stable in an acidic environment. The drug inhibits the synthesis of bacterial cell walls and acts both locally and systemically after absorption into the bloodstream and subsequent penetration into the lumen of the stomach. H. pylori demonstrates good sensitivity to amoxicillin in vitro, but complex therapy is required to eradicate the bacterium.
Clarithromycin
Clarithromycin, a 14-member macrolide, is a derivative of erythromycin with a similar spectrum of activity and indications for use. However, unlike erythromycin, it is more acid resistant and has a longer half-life. The results of studies showing that a triple anti-Helicobacter therapy regimen using clarithromycin gives a positive result in 90% of cases has led to the widespread use of the antibiotic. In this regard, an increase in the prevalence of clarithromycin-resistant strains of H. pylori has been recorded in recent years.
Tetracyclines
The point of application of tetracyclines is the bacterial ribosome. The antibiotic interrupts protein biosynthesis and specifically binds to the 30S ribosomal subunit, eliminating the addition of amino acids to the growing peptide chain. Tetracycline has been shown to be effective in vitro against H. pylori and remains active at low pH.
Proton pump inhibitors (PPIs)
PPI therapy has proven effective in various clinical studies. It is assumed that antisecretory drugs of the PPI group may help increase the concentration of antimicrobial agents, in particular metronidazole and clarithromycin, in the gastric lumen. PPIs reduce the volume of gastric juice, as a result of which the leaching of antibiotics from the surface of the mucosa decreases and the concentration, accordingly, increases. In addition, reducing the volume of hydrochloric acid maintains the stability of antimicrobials. Different PPIs do not differ in effectiveness.
Metronidazole
H. pylori is generally very sensitive to metronidazole, the effectiveness of which is independent of pH. After oral or infusion use, high concentrations of the drug are achieved in the gastric juice, which makes it possible to achieve the maximum therapeutic effect. Metronidazole causes H. pylori to lose its helical DNA structure, causing DNA damage and killing the bacterium.
Bismuth preparations
Bismuth was one of the first drugs to eradicate H. pylori. There is evidence that bismuth has a direct bactericidal effect, although its minimum inhibitory concentration (MIC - the smallest amount of drug that inhibits the growth of a pathogen) against H. pylori is too high. Like other heavy metals such as zinc and nickel, bismuth compounds reduce the activity of the enzyme urease, which is involved in the life cycle of H. pylori. In addition, bismuth preparations have local antimicrobial activity, acting directly on the bacterial cell wall and disrupting its integrity.
Diagnostics
To detect infection in the body, various examination methods are used, each of which has its own advantages, disadvantages and limitations. Traditionally, all methods are divided into non-invasive and invasive.
Invasive detection methods:
- Histological examination - examination of specially stained samples of stomach tissue obtained through biopsy during endoscopic examination under a microscope.
- Microbiological seeding and isolation of Helicobacter bacteria. To obtain material for culture, a biopsy or a sample of gastric juice is used, which is obtained during an endoscopic examination.
- Polymerase chain reaction (PCR) - can detect infection in small tissue samples obtained through biopsy.
- Rapid urease test - this method uses the ability of bacteria to process urea. The tissue sample obtained by biopsy is placed in a medium containing urea and a pH indicator. Bacteria break down urea into carbon dioxide and ammonia, which increases the pH of the medium and changes the color of the indicator.
Non-invasive detection methods:
- Serological blood tests that can detect antibodies to Helicobacter.
- Urea breath test. During this examination, the patient is given a solution with urea, the molecule of which contains a labeled carbon isotope, to drink. Helicobacter breaks down urea into ammonia and carbon dioxide, which contains a labeled carbon atom. This gas enters the bloodstream and is expelled through the lungs with air. Half an hour after consuming the urea solution, the patient exhales into a special bag, in which a labeled carbon atom is detected using spectrometry.
- Detection of H. pylori antigens in stool.
How to get rid of Helicobacter pylori?
In 2020, an acceptable Helicobacter pylori eradication regimen in adults is considered to be a treatment regimen that provides at least 80% of effectively confirmed by repeated examination cure of Helicobacter pylori infection and healing of ulcers or gastritis, lasting no more than 14 days and having acceptably low toxicity (side effects should develop in no more than 10-15% of patients and in most cases not be so serious as to require early cessation of treatment).
New schemes and protocols for Helicobacter eradication are constantly being developed. This serves several purposes:
- increasing the convenience of treatment for patients and the degree of their compliance with the treatment regimen: eliminating the need for a strict “anti-ulcer” diet
- thanks to the use of powerful proton pump inhibitors;
- reducing the duration of treatment (from 14 to 10, then 7 days);
- reducing the number of drugs taken simultaneously through the use of combination drugs;
- reducing the number of doses per day due to the use of prolonged forms of drugs or drugs with a long half-life (T1/2);
- reducing the likelihood of unwanted side effects;
- overcoming the growing resistance of Helicobacter to antibiotics;
- meeting the need for alternative treatment regimens if there is an allergy to any of the components of the standard regimen or if the initial treatment regimen has failed.
In 2020, Maastricht IV experts recommended the following Helicobacter pylori eradication regimens:
Treatment regimen recommended at the Maastricht-IV conference
Triple therapy was proposed at the first Maastricht conference and has become a universal treatment regimen for H. pylori infection. It is recommended by all world conciliation conferences.
The regimen includes drugs:
- one of the proton pump inhibitors (PPIs) in a “standard dosage” (omeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, esomeprazole 20 mg, or rabeprazole 20 mg twice a day) for at least 7 days
- Clarithromycin (500 mg 2 times a day) 7 days
- amoxicillin (1000 mg 2 times a day) or metronidazole (500 mg 2 times a day) 7 days.
It has been shown that the regimens of PPI + clarithromycin + metronidazole (tinidazole) and PPI + clarithromycin + amoxicillin are equivalent. It has been established that the effectiveness of triple therapy increases when its duration is increased to 10 or 14 days (depending on the degree of Helicobacter pylori contamination and the patient’s tolerance to therapy).
Treatment regimen recommended by the Russian Society of Gastroenterologists
Due to different resistance to antibiotics in different regions of the world, the prevalence of different strains of Hp, and the genetic characteristics of the population, different countries or groups of countries develop their own recommendations regarding the eradication of Hp. Some of these parameters, in particular HP resistance to certain antibiotics, change over time. The choice of a specific regimen is also determined by the patient’s individual intolerance to the drugs, as well as the sensitivity of the HP strains with which the patient is infected.
At the congress of the Scientific Society of Gastroenterologists of Russia, the following HP eradication schemes were adopted; they are relevant for 2019:
1) First option. Three-component therapy, including the following drugs, which are taken for 10-14 days:
- one of the PPIs in a “standard dosage” 2 times a day +
- amoxicillin (500 mg 4 times a day or 1000 mg 2 times a day) +
- clarithromycin (500 mg 2 times a day), or josamycin (1000 mg 2 times a day) or nifuratel (400 mg 2 times a day).
2) Second option. Four-component therapy, including in addition to the drugs of option 1 bismuth drug, its duration is also 10-14 days:
- one of the PPIs in a “standard dosage” +
- amoxicillin (500 mg 4 times a day or 1000 mg 2 times a day) +
- clarithromycin (500 mg 2 times a day), or josamycin (1000 mg 2 times a day), or nifuratel (400 mg 2 times a day) +
- bismuth tripotassium dicitrate 120 mg 4 times a day or 240 mg 2 times.
3) Third option. If the patient has atrophy of the gastric mucosa with achlorhydria confirmed by intragastric pH-metry and it is therefore inappropriate to prescribe acid-suppressing drugs (PPN or H2 blockers), the third option is used (lasting 10-14 days):
- amoxicillin (500 mg 4 times a day or 1000 mg 2 times a day) +
- clarithromycin (500 mg 2 times a day), or josamycin (1000 mg 2 times a day), or nifuratel (400 mg 2 times a day) +
- bismuth tripotassium dicitrate (120 mg 4 times a day or 240 mg 2 times a day).
4) Fourth option. If full eradication therapy is not possible for elderly patients, truncated regimens are used:
- one of the PPIs in a “standard dosage” +
- amoxicillin (500 mg 4 times a day or 1000 mg 2 times a day) +
- bismuth tripotassium dicitrate (120 mg 4 times a day or 240 mg 2 times a day).
Another way: bismuth tripotassium dicitrate 120 mg 4 times a day for 28 days. If you have pain in the stomach, take a short course of PPI.
Eradication of Helicobacter pylori infection using first-line triple therapy
Introduction
The Maastricht Consensus III 2005 confirmed the need for eradication of infection in patients with Helicobacter pylori
associated gastroduodenal ulcers.
The destruction of Helicobacter pylori infection gives a chance to cure the disease, that is, to eliminate recurrences of the ulcer in the future. as first choice
for 7-14 days: standard dosage proton pump inhibitor twice daily + clarithromycin 500 mg twice daily + amoxicillin 1000 mg twice daily or metronidazole 500 mg twice daily . If the primary resistance of Helicobacter pylori (H. pylori) to metronidazole is above 40%, this drug is not recommended for use in a triple eradication therapy regimen.
Studies conducted in Russia have shown that primary resistance to metronidazole exceeds 50%
.
It can be assumed that a similar situation exists in our country. In Western Europe, where primary resistance to metronidazole is low, triple therapy protocols with metronidazole have been reported to be highly effective. In recent years, a new nitroimidazole derivative, ornidazole
(Dazolik), has appeared, which has advantages over metronidazole. Pharmacokinetic advantages include a longer half-life of the drug (ornidazole - 13 hours, metronidazole - 7 hours); lack of interaction with the cytochrome P450 enzyme system, better penetration through the bacterial wall. Ornidazole does not cause inhibition of acetaldehydrogenase and the appearance of disulfiram-like reactions, which allows alcohol intake during treatment. Thanks to this, adherence to pharmacotherapy may increase and the range of patients in whom the drug can be used can expand. There are reports of a fairly high eradication rate (85.7%) when using the ornidazole protocol. The effectiveness of such a protocol has not been studied in our region.
Goal of the work
consisted of a comparative evaluation of the effectiveness of the first-line treatment protocols for H. pylori infection: proton pump inhibitor + clarithromycin + amoxicillin and proton pump inhibitor + ornidazole.
Patients and research methods
The results of two options for eradication therapy were studied in a randomized study in patients in the Vitebsk region. The methodological rules for eradication were carefully followed. The purpose, objectives, possible results and side effects of eradication therapy were explained to all patients in detail, and motivation for treatment was created. 47 patients with H. pylori infection and erosive or ulcerative lesions of the stomach and/or duodenum were examined and treated. All patients were constantly taking non-steroidal inflammatory drugs. The age of the subjects was 22-60 years.
Determination of H. pylori infection before treatment was carried out with the simultaneous use of two diagnostic methods: morphological examination of gastrobiopsy
(staining with hematoxylin-eosin according to Giemsa and Alcian blue, 2 gastrobiopsy specimens were obtained from the antrum and 2 from the body of the stomach) and
the rapid urease method
with test kits from the Semper Unitary Enterprise (Republic of Belarus).
8 weeks or later after eradication treatment, but not earlier than 4 weeks after cessation of antisecretory or any antibacterial therapy, H. pylori was re-diagnosed with a rapid urease test. Successful eradication was determined by a negative result of the rapid urease test.
Two options for eradication therapy were used
:
- OKA protocol
- one-week triple therapy (25 patients) with Peptipak drug sets: 20 mg omeprazole, 500 mg clarithromycin and 1000 mg amoxicillin; each of the drugs 2 times a day; - LKO protocol
- triple therapy (22 patients) according to the following protocol: 30 mg of lansoprazole, 500 mg of clarithromycin and 1000 mg of ornidazole; each of the drugs - 2 times a day.
In the group of patients treated according to the LKO protocol, the following drugs were used: ornidazole - “Dazolik” (India), lansoprazole (MaxPharma, Cyprus); clarithromycin - "Cleron" (MaxPharma, Cyprus). In the case of the OKA protocol, a set of drugs “PeptiPac” was used, which included omeprazole, clarithromycin and amoxicillin, designed for one week of treatment (Jerusalem).
Main analyzed indicators
when assessing the effectiveness of treatment:
- intention to treat
(ITT) - the percentage of patients cured (freed from Helicobacter pylori infection) and the total number of patients who started treatment; - per protocol
(PP) - the percentage of cured patients and the number of those who completed treatment in full in strict accordance with the protocol. Only those patients who underwent surveillance endoscopy were taken into account; 8 patients refused re-examination, citing the absence of any gastroenterological complaints.
To assess differences in numerical values, the nonparametric goodness-of-fit test χ2 for contingency tables (Pearson-Fisher test) was used.
Results and discussion
The results of the study of H. pylori eradication are given in table. 1. According to the rapid urease test, when using the classic first-line protocol and its modifications in terms of intention to treat, eradication was achieved in 84.0-85.7%, per protocol - in 85.7-87.5% of cases. No statistically significant differences were found between the OKA and LKO protocols (according to per protocol χ2 = 0.12; p > 0.1); both treatment options have proven to be effective.
Table 1. Results of eradication therapy
| Protocol | Number of patients | Eradication rate | ||||
| intention to treat | per protocol | |||||
| intention to treat | per protocol | abs. | % | abs. | % | |
| OKA | 25 | 24 | 21 | 84,0 | 21 | 87,5 |
| LKO | 14 | 14 | 12 | 85,7 | 12 | 85,7 |
Six out of 25 patients reported the following side effects
when using the OKA protocol:
- nausea - 1 person,
- bitterness and dry mouth - 1 (in the same patient who had nausea),
- loosening of stool (increase in frequency by 1 time and/or change in stool shape to type 5-6 on the Bristol scale) - 4,
- severe diarrhea leading to cessation of treatment - 1 patient.
Side effects when using
noted in 10 out of 22 patients, and the effects were often combined in the same patient:
- nausea - 3,
- single vomiting - 1,
- bitterness in the mouth - 3,
- dry mouth - 2,
- loose stool – 5,
- diarrhea - 2 cases.
The differences in the number of patients with side effects in the two groups were statistically insignificant
: χ2 = 1.54; p > 0.05.
The presented results correspond to the results of modern Western European and North American randomized studies, in which the effectiveness of treatment was monitored by a breath test with urea containing 13C. Previously, we conducted a meta-analysis of publications over 5 years (1997-2002) on the effectiveness of eradication therapy in 3226 patients using the OCA regimen in dosages recommended by the Maastricht consensus - II. According to the results of the meta-analysis, it was found that the average value of eradication by intention to treat was 78.6, per protocol - 83.2%. The results obtained differ significantly from the results of treatment in the early 1990s, when the eradication efficiency was 90-98%
. Considering that our study is ongoing, it is possible to adjust the final results in the direction of reducing the frequency of eradication, which is a more adequate result of treatment in our conditions.
There are about 50 meta-analyses regarding the problems of eradication therapy. An important feature of assessing the results of eradication therapy is strict adherence to methodological rules. Ignoring any of these rules, for example, conducting an eradication assessment during or shortly after the cessation of antisecretory and/or antibacterial therapy, gives false-positive results of successful eradication and subsequently an erroneously high rate of reinfection. A practicing doctor should not deviate from generally accepted treatment protocols, independently “improving” them.
According to the Maastricht Consensus III 2005, a fourteen-day treatment duration is 12% more effective than a seven-day treatment duration
(95% confidence interval 7-17%). Seven-day therapy may be used in cases where local studies have shown its effectiveness. The proton pump inhibitor-clarithromycin-amoxicillin or metronidazole protocol is recommended as first-line therapy in populations with clarithromycin resistance below 15-20%. In populations with metronidazole resistance below 40%, the proton pump inhibitor-clarithromycin-metronidazole protocol should be preferred. Quad therapy is a possible alternative as a first choice protocol. The last provision is new from the point of view of normative statements, although it was previously used in practice.
Failure of the first line of treatment requires, in accordance with the Maastricht Consensus II and III, the use of a second line protocol
: proton pump inhibitor at a standard dose 2 times a day + bismuth subcitrate/salicylate 120 mg 4 times a day + metronidazole 500 mg 3 times a day + tetracycline 500 mg 4 times a day; Duration of treatment is 7-14 days. In the recommendations of the Maastricht Consensus - III 2005.
The last statement regarding second-line treatment cannot be considered fully justified. There is a return to the proton pump inhibitor-amoxicillin-metronidazole protocol rejected by Maastricht II. True, earlier there was talk of using this combination as the first line of therapy. It is hardly advisable to recommend a proton pump inhibitor-amoxicillin-metronidazole protocol in our population. In a Russian randomized multicenter study, V. T. Ivashkin and co-authors studied the level of eradication when using omeprazole (20 mg 2 times a day) + amoxicillin (1000 mg 2 times a day) + metronidazole (400 or 500 mg 2 times a day) and obtained extremely low eradication rate - 30%
(95% confidence interval 17-43%).
When recommending the proton pump inhibitor-amoxicillin-metronidazole protocol, the consensus materials cite only one randomized trial. Notably, in the next consensus paper cited, the eradication rate using the above regimen was 57%, but the metronidazole resistance rate was only 30%. These data confirm that the use of such a treatment protocol will give even less effect. When using the proton pump inhibitor-tetracycline-metronidazole eradication protocol, a proton pump inhibitor is used in a standard dose 2 times a day + metronidazole 500 mg 2 times a day + tetracycline 500 mg 4 times a day. It is hardly advisable to apply this protocol in our population without reliable data (randomized studies) on the effect of treatment in conditions similar to ours, at least in terms of infection and primary antibacterial resistance. The “unavailability” of colloidal bismuth preparations mentioned in the consensus materials is associated with a ban on their use in certain countries.
If the second line of eradication is ineffective, third line treatment
(“third choice”) As noted in the latest consensus, “third-line treatment should be based on antimicrobial susceptibility testing of H. pylori.” In our conditions today this is impossible. Abroad, levofloxacin or rifabutin are sometimes included as one of the third-choice protocol drugs. Typically, levofloxacin (500 mg twice daily) + proton pump inhibitor (standard dose twice daily) + amoxicillin (1000 mg twice daily) is used in triple therapy, lasting 7 or 10 days. The per protocol eradication rate of such treatment, according to a systematic review, is 63-86%. For economic reasons, the use of protocols with levofloxacin on a mass scale is hardly advisable in our country.
What to do if eradication is unsuccessful after using triple therapy as the first line and quadruple therapy as the second line? One of the leading consensus experts, Secretary of the European H. pylori Study Group (EHSG), Professor F. Megro makes a number of recommendations in one of his articles. Our randomized trial and daily clinical experience confirm the validity of such recommendations and somewhat complement them.
So, if previous treatment is ineffective
you should choose a triple therapy option that differs in the combination of antibiotics and extend the treatment time to 2 weeks.
It is possible to use a new version of quadruple therapy from among those recommended. Another way to increase the effectiveness of eradication is to replace the proton pump inhibitor with a more effective one. For our country, the problem of a reliable generic is of paramount importance. During treatment, the patient should be advised to take kefir or yogurt
containing bifidobacteria and lactobacilli.
This will prevent the development of antibiotic-associated diarrhea, which rarely causes treatment refusal. There is evidence that the inclusion of Lactobacillus acidophilus, Lactobacillus gasseri, Saccharomyces boulardii and Bifidobacterium
improves eradication results.
We should not forget about the patient's adherence to pharmacotherapy. The need to abstain from alcohol should be discussed in advance with the patient, since if this condition is not met, metronidazole or furazolidone cannot be taken. Smoking
has been shown to be associated with a reduced eradication effect.
Conclusion
According to the urease test, during one-week triple eradication therapy of the first line according to the proton pump inhibitor-clarithromycin-ornidazole
eradication occurs in 85.7% of patients who received full treatment, and when using the
proton pump inhibitor-clarithromycin-amoxicillin
- in 87.5% of patients, which confirms the sufficient effectiveness of both treatment protocols.
Pimanov S. I., Makarenko E. V., Ovchinnikov V. V., Kavtsevich M. L., Koroleva Yu. I., Semenova E. V., Sapego L. G.
Vitebsk State Medical University, Vitebsk Regional Clinical Hospital. Published: “Medical Panorama” No. 5, April 2008.
Possible complications from antibiotic treatment
Factors that increase the risk of side effects during eradication therapy:
- Individual intolerance to drugs;
- Presence of somatic pathologies;
- Negative state of intestinal microflora during the initial period of treatment.
Complications of eradication therapy – side effects:
- An allergic reaction to drug components that disappears after discontinuation;
- Dyspeptic symptoms of the gastrointestinal tract (discomfort in the stomach and intestines, taste of bitterness and metal, nausea and vomiting, diarrhea, flatulence). Usually all these phenomena disappear spontaneously after a short time. In rare cases (5-8%), the doctor prescribes medications against vomiting or diarrhea, or cancels the course.
- Dysbacteriosis. It more often manifests itself in patients who previously had gastrointestinal dysfunction, and develops during treatment with tetracycline drugs or during therapy with macrolides. A short-term course is not able to upset the balance of intestinal microflora; to prevent dysbiosis, you need to consume fermented milk products more often: yogurt, kefir.
Nutrition and diet
Of course, the main point in the treatment of this pathology is taking medications, but proper nutrition plays an equally important role. To easily get rid of helicobacteriosis, you should follow the following recommendations:
- do not take long intervals between meals;
- eat food in small portions;
- follow a diet of 5-6 meals a day, eating slowly, chewing food well and drinking it with enough liquid;
- the patient should avoid excessively fatty, fried or spicy foods, carbonated drinks, pickled foods, and alcohol.
In reality, these are only general recommendations; in each individual case, nutrition should be calculated based on the level of acidity (low, high) and prescribed only by the specialist conducting the treatment.
Prevention
You can completely recover from helicobacteriosis if, in addition to therapy, you adhere to preventive measures:
- Maintaining hygiene. Wash your hands before eating, do not consume dirty vegetables and fruits, or questionable water. Do not use other people's household items.
- Timely detection of the disease. If you feel unwell or suspect the presence of a pathogenic bacterium in the body, it is important to immediately consult a doctor and undergo the necessary tests.
- Strengthening the immune system. A healthy lifestyle (swimming, running, walking) increases your defenses and prevents pathogens from entering the body.
- Proper nutrition. Small meals, small doses and avoidance of fried, salty, spicy, smoked foods, alcohol and smoking.
The main danger of helicobacter pylori is that it can provoke gastritis, ulcers, even malignant neoplasms. It is impossible to get rid of harmful bacteria without antibiotics. Therefore, it is important to strictly adhere to special treatment regimens and observe preventive measures.